What do cats, dogs, horses, and ticks have in common? Allergenicity and component resolved diagnostics
For a printable version of the June CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the clinical history of allergy testing.
2. Describe what component resolved diagnostics is and how it differs from whole allergen testing.
3. Describe the protein families for pet allergens and their impact on clinical strategies to manage allergic sensitization.
4. Recall what Alpha-Gal syndrome is and how it is diagnosed through in vitro testing.
A young girl asks her parents for a pet, perhaps a dog or cat. The parental answer is, “No, your brother is allergic.” This is a common scenario, as up to 60% of Americans own a domesticated pet.1 Through diagnostic testing, this answer could be different. With the use of blood allergy testing and component resolved diagnostics (CRD), answers could be, “Sure, we can get a female dog instead of a male dog.” Another answer could be, “Yes, we can get a dog, but not a cat, because we know your brother is only allergic to cats, not dogs.”
In another scenario, a patient presents to the emergency room late in the evening with a symptomatic allergic reaction to something, but the patient’s medical history is unclear about exposure to specific allergens. In this case, too, allergy diagnostics help complement a patient’s history and aid in the diagnosis and management of allergic diseases.
How allergy diagnostics help
An in vitro (blood) whole allergen test can identify sensitization, but just as with many other laboratory tests, there is more to the story. A whole allergen is made up of a variety of proteins, and these proteins may or may not have an association with clinical symptoms. Within the whole allergen, there may be a variety of proteins, some of which may be cross-reactive, which means they have a similar protein structure to other clinically non-relevant proteins. Other proteins may be unstable to heat or digestion, or they may be species-specific proteins that can drive clinical symptoms and are often associated with significant risk for systemic reactions.
The use of specific IgE (sIgE) tests aid in the diagnosis of allergen sensitization alongside a thorough patient history. If the whole allergen is positive via in vitro blood testing or skin prick testing, additional in vitro blood testing is often available to tell “the rest of the story.” This is known as allergen component resolved diagnostics (CRD).
History of allergy testing
Tracing the history and evolution of allergy testing shows the trajectory of allergen diagnostics. Skin testing dates back to 1865, when Charles H. Blackley, a physician who suffered from allergic rhinitis, abraded his skin with a lance, then applied grass pollen to the wound, resulting in intense itching and a large cutaneous response.2 This scratch testing procedure led to the first applied skin prick tests (SPT)3 by Lewis and Grant in 1924.
In 1967, Immunoglobulin E was the last of the five immunoglobulins (IgG, IgM, IgD, IgA) to be discovered and was attributed to type I hypersensitivity allergic reactions.4,5 Blood allergy testing became commercially available through radioallergosorbent (RAST) testing in 1974. Improvements to this method of testing came in 1981, with the introduction on an enzyme linked assay. Currently, there is a third generation of immunofluorescence5 testing, which binds allergens to a 3-dimensional polymer sponge, allowing for superior surface binding.6
Once components for milk and egg became commercially available in the United States, this immunofluorescence method and instrumentation expanded to allergen component resolved diagnostics in 2005.7 Allergen components have been extensively researched and have been available globally for over two decades. CRD will continue to advance well into the future, as molecular allergology continues to enhance the diagnosis of allergic disease.
Both whole allergen and component allergen testing are sandwich IgE assays, allowing for solid phase binding of the relevant and specific antibodies. Therefore, it is imperative to have reliable source material bound to the sponge or well; this is done utilizing either native purified proteins or recombinant DNA technology.6 However, skin prick testing for allergen components is not readily available. The ease of in vitro blood testing only requires a venipuncture and provides fully quantitative measurement and reproducibility of results.6
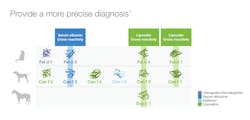
Previously, in the July 2020 issue of Medical Laboratory Observer, Lakiea Wright, MD, noted that component testing is emerging as critical indicator for anaphylactic risk through the use of available food allergen components and stinging insect components.8 Listed in Figure 1, there are examples of many clinically relevant allergen components currently accessible to laboratories and clinicians that have been approved by the U.S. Food and Drug Administration (FDA). Several allergen components are available in the categories of peanut and tree nuts, pets, stinging insects, and even a carbohydrate component called alpha-Gal.Automation and CRD within the lab
Using automated instruments with high walk away times maximizes efficiency of CRD, as the calibration, quality control, and curve control are all in-house, except for carriers, allowing for lab efficiency.
Fully automated reflex testing with whole allergen positivity puts orders back on track, allowing a faster turnaround time for component allergen results. Automation also provides flexibility by being able to batch and run testing daily, as well as offering different ways to run the instrument. Workflow can be enhanced through optional automated track connectivity and integrative-operation software systems. This type of laboratory automation integrates sample handling to improve efficiency, quality, and safety across various analyzers in the laboratory.9
In addition, component resolved diagnostic testing is reimbursed at three times the whole allergen rate.10 Keeping this testing within the laboratory, as opposed to a more costly and time consuming send out, is beneficial to patients, gives providers clarity on treatment options, and makes the laboratory more efficient and valuable. A collaborative initiative with all relevant stakeholders allows for the appropriate use of allergy CRD and increased opportunities for value-based testing.
Value to patients
Now that allergen components have been defined, what is the value to the patient? In the scenario described about a family wanting to obtain a pet, but being limited because of a child’s allergic symptoms, CRD can aid in that patient’s dilemma. Pet allergen components can help define a primary allergen sensitization that is likely responsible for clinical symptoms, versus an irrelevant cross reactivity with other pets. In the case of dogs, CRD can help determine the sex of the pet that a patient may tolerate.
Domestication of animals has turned what were once exclusively outdoor allergens into indoor allergens, increasing the cumulative exposure to mammalian aeroallergens, specifically to dogs and cats. For those who are sensitized and have clinical symptoms, this is problematic. Symptoms for respiratory diseases, such as asthma and rhinitis, can complicate the cohabitation and range from mild to life threating asthma exacerbations.
Dog, cat and horse component resolved diagnostics have been widely studied and demonstrate the positive impact they can have on the management of patients, up to and including the ability to predict severity and development of asthma and rhinitis.11 Polysensitization to pet allergen components has been correlated with increased bronchial inflammation in those with severe asthma.1,11,12 Availability of CRD offers more personalized diagnostic work-ups, which facilitates identification of primary sensitization, versus cross reactive sensitizations between animal species.
Proteins of pet allergens
Protein families for relevant pet allergens can be classified into secretoglobins, kallikreins, lipocalins and serum albumins.12 These allergens are present in pet dander, saliva and urine, and are distributed into the environment through shedding.12,13 The protein sequences of many allergen components are unique, but some lipocalins and serum albumins share sequence homology, which can cause cross-reactivity.
Secretoglobins (also known as uteroglobins) most commonly refer to Fel d 1 produced in the saliva of cats. This highly allergenic component is the cause of most allergic clinical symptoms and is structurally unique; therefore, if a patient is symptomatic with cats and is sensitized to Fel d 1, the Fel d 1 is considered a primary sensitizer.12
Another unique pet component family is kallikrein. This unique prostatic kallikrein protein is produced in male dogs. This specific primary protein is expressed in the prostate and shed in saliva and dog dander.1 About 30% of patients who are monosensitized to dog are sensitized to Can f 5; therefore, they may tolerate a female dog or castrated male dog.12 However, this is not the only significant dog allergy component.
Lipocalins are a significant allergen protein family and can be a primary allergen sensitizer or cross-reactive allergen between the various pets, such as cat, dog, and horse.1,13 These allergens are expressed in saliva and dander, regardless of breed or supposed hypoallergenicity.1 See Figure 2. Lastly, serum albumins are highly cross-reactive molecules, abundant in dander and saliva, and play little role with clinical symptoms.1
Evaluating a patient’s pet allergens
Evaluating the patient’s unique pattern of pet components allows more precise evaluation of the primary sensitizing allergen causing the patient’s symptoms. With protein homology between certain pet families, a whole allergen to dog, cat or horse may be positive, when, in fact, the problem is really cross-sensitization to another animal species. This CRD of primary species sensitization versus cross reactivity information directly impacts patient management.1
The pet management consideration informs evidence-based recommendations, such as should there be avoidance of cat, dogs and/or horses? Would a female dog be a better option? Would a cat be a better choice for a family than a dog?
But pet selection or re-homing a pet is not the only consideration. Those sensitized to three or more pet allergen components are at risk for development or worsening of respiratory illnesses. By having this understanding, correct pet avoidance, appropriate medications, and allergen immunotherapy can reduce the bronchial hyperresponsiveness.11
Although elimination of pets from the home is considered the most effective avoidance measure, this is often emotionally charged and not a viable option. Novel approaches to reducing the expression of allergenic proteins from pets are emerging. Recently, a cat food that binds with the major allergenic protein Fel d 1, reducing its expression in cats, has been introduced.14 In addition, other therapeutic interventions for both pets and humans affected by pet allergy have been studied and may hold promise for the future.
For the family inquiring about a pet, these precision diagnostics can lead to the most appropriate pet selection and targeted management for the patient.
Alpha-Gal syndrome
In a different clinical scenario, when a patient has a systemic allergic reaction and medical history is unclear, consideration of alpha-Gal syndrome may be appropriate. Alpha-Gal syndrome is a mammalian (red) meat allergy, typically presenting as a delayed systemic reaction or anaphylaxis, caused by sensitization to a sugar complex protein, galactose-α-1,3-galactose (α-Gal), which is an oligosaccharide present in all mammals, except humans and Old-World monkeys.15,16
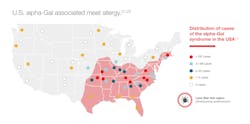
The vector responsible for sensitization to alpha-Gal is a tick from the family Ixodidae. In the United States, the Lone Star tick (Ambylomma americanum) is responsible for the majority of alpha-Gal sensitization.17,18 Incidence correlates with geographic distribution of the tick: see Figure 3. Transmission occurs with certain tick species that have alpha-Gal present in the intestine from a previous mammalian host that transmits this sugar complex to the human after tick bite.17
The response to this foreign oligosaccharide results in a type I hypersensitivity and antibody production to alpha-Gal. The delay in response to this sugar complex exposure in mammalian meat is due to absorption of alpha-gal-containing glycolipids binding to lipid particles, which take hours to form and be released into the circulation.17 Therefore, it may result in a delayed allergic response, following ingestion of mammalian meat.15
Diagnosis of alpha-Gal syndrome can be elusive. Alpha-Gal syndrome is often found in adults who had no previous complaints of meat allergy. Symptoms have also been reported after exposure to certain alpha-Gal containing medications and gelatin containing foods.18 Diagnosis can be challenging, as this is a delayed hypersensitive response to mammalian meat, typically presenting 3-6 hours after ingestion.15,17,18 Therefore, identification of the offending trigger can be difficult for patients and clinicians to pinpoint.
In the patient scenario previously mentioned, a patient presents to the emergency room late in the evening with a symptomatic allergic reaction to an unknown trigger, and the history is unclear for exposure to allergens. In endemic areas, alpha-Gal is a likely cause, warranting further exploration of a patient’s medical history and alpha-Gal sIgE diagnostic testing.
In-vitro testing
In-vitro testing for alpha-Gal syndrome is available with the measurement of IgE antibodies against alpha-Gal, along with immunoassay testing for sIgE to beef, lamb, and pork. Often, a profile for alpha-Gal will also include tryptase and a total IgE. This component profile optimized for clinicians to evaluate a difficult diagnostic presentation is valuable and helps inform both diagnosis and patient management.
While levels of alpha-Gal can decrease over time, subsequent tick bites can increase the levels and the clinical symptoms when mammalian meat is ingested. Avoidance of tick exposure and bites is recommended, and avoidance of mammalian meats is the mainstay of management to prevent future life-threatening reactions.15,18 Often, patients will tolerate chicken, turkey, or fish without symptoms.15 They also should use caution with gelatin containing products and certain medications.18,19
Other management considerations for alpha-Gal syndrome are an epinephrine auto-injector for possible systemic reactions and consideration about using an oral food challenge to determine risk.20
The future of allergic disease diagnosis will continue to be personalized with component resolved diagnostics. This approach and the availability of additional in vitro specific IgE allergen components allows an efficient and confident health system approach to allergic disease management.
References
- Matricardi PM, et al EAACI Molecular allergology user’s guide. Pediatr Allergy Immunol. 2016: 27: (suppl23): 1–250. https://doi.org/10.1111/pai.12563.
- Blackley CH. Experimental Researches on the Causes and Nature of Catarrhus Aestivus. London; Balliere; 1873. [Google Scholar]
- Bousquet J, Michel FB. Precision of prick and puncture tests. J Allergy Clin Immunol. 1992;90(6 Pt 1):870-872. doi:10.1016/0091-6749(92)90458-e.
- Platts-Mills TA, Heymann PW, Commins SP, Woodfolk JA. The discovery of IgE 50 years on. Ann Allergy Asthma Immunol. 2016;116(3):179–182. doi:10.1016/j.anai.2016.01.003.
- Allergy and Autoimmune Disease Diagnostics. Thermo Fisher Scientific. http://www.phadia.com/en/About-us/Phadia-History/. Accessed May 18, 2021.
- Popescu FD, Vieru M. Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules. World J Methodol. 2018 Nov 29;8(3):17-36. doi: 10.5662/wjm.v8.i3.17. PMID: 30519536; PMCID: PMC6275558.
- U.S. Department of Health & Human Services. 510(k) Premarket notification. U.S. Food and Drug Administration. Published June 9, 2005. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K051218. Accessed May 25, 2021.
- Wright L. Component testing emerging as critical indicator for anaphylactic risk. Medical Laboratory Observer. July 2020. https://www.mlo-online.com/continuing-education/article/21142397/component-testing-emerging-as-critical-indicator-for-anaphylactic-risk. Accessed May 18, 2021.
- Phadia immunology reference laboratory. Thermo Fisher Scientific - US. https://www.thermofisher.com/us/en/home/clinical/diagnostic-testing/condition-disease-diagnostics/allergy-autoimmune-disease-diagnostics/allergy-diagnostics/phadia-immunology-reference-laboratory.html. Accessed May 18, 2021.
- CPT Code - Qualitative or Semiquantitative Immunoassays 86000-86804 - Codify by AAPC [Internet]. Aapc.com. 2021 [cited 2021 Jan 17]. https://www.aapc.com/codes/cpt-codes-range/276/. Accessed May 19, 2021.
- Nordlund B, Konradsen JR, Kull I, Borres MP, Önell A, Hedlin G, Grönlund H. IgE antibodies to animal-derived lipocalin, kallikrein and secretoglobin are markers of bronchial inflammation in severe childhood asthma. Allergy. 2012 May;67(5):661-9. doi: 10.1111/j.1398-9995.2012.02797.x. Epub 2012 Feb 17. PMID: 22339365.
- Schoos AM, Nwaru BI, Borres MP. Component-resolved diagnostics in pet allergy: Current perspectives and future directions. J Allergy Clin Immunol. 2021 Jan 11:S0091-6749(21)00003-8. doi: 10.1016/j.jaci.2020.12.640. Epub ahead of print. PMID: 33444632.
- Zahradnik E, Raulf M. Respiratory Allergens from furred mammals: environmental and occupational exposure. Vet Sci. 2017;4(3):38. Published 2017 Aug 4. doi:10.3390/vetsci4030038.
- Matulka RA, Thompson L, Corley D. Multi-level safety studies of Anti Fel d 1 IgY ingredient in cat food. Front Vet Sci. 2020 Jan 8;6:477. doi: 10.3389/fvets.2019.00477. PMID: 31970163; PMCID: PMC6960183.
- Wolver, Susan E et al. “A peculiar cause of anaphylaxis: no more steak? The journey to discovery of a newly recognized allergy to galactose-alpha-1,3-galactose found in mammalian meat.” J Gen Intern Med. 28,2 (2013): 322-5. doi:10.1007/s11606-012-2144-z.
- Macher BA, Galili U. The Galα1, 3Galβ1, 4GlcNAc-R (α-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochimica Biophysica Acta (BBA)-General Subjects. 2008;1780(2):75-88.
- Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, Cooper PJ, Platts-Mills TA. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. J Allergy Clin Immunol. 2011 May;127(5):1286-93.e6. doi: 10.1016/j.jaci.2011.02.019. Epub 2011 Mar 31. PMID: 21453959; PMCID: PMC3085643.
- Platts-Mills TAE, Li RC, Keshavarz B, Smith AR, Wilson JM. Diagnosis and management of patients with the α-Gal Syndrome. J Allergy Clin Immunol Pract. 2020 Jan;8(1):15-23.e1. doi: 10.1016/j.jaip.2019.09.017. Epub 2019 Sep 28. PMID: 31568928; PMCID: PMC6980324.
- Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-α-1,3-galactose. J Allergy Clin Immunol. 2012 May;129(5):1334-1342.e1. doi: 10.1016/j.jaci.2012.02.038. Epub 2012 Apr 3. PMID: 22480538; PMCID: PMC3340561.
- Wilson JM, Platts-Mills TAE. α-Gal and other recent findings that have informed our understanding of anaphylaxis. Ann Allergy Asthma Immunol. 2020 Feb;124(2):135-142. doi: 10.1016/j.anai.2019.11.024. Epub 2019 Nov 28. PMID: 31785367; PMCID: PMC7318893.
- Adapted from Platts-Mills T. The Alpha-gal Syndrome: IgE responses to galactose alpha-1,3-galactose induced by bites from lone star ticks. Presentation presented at AAAI; 2019.
- Regions where ticks live. Centers for Disease Control and Prevention. https://www.cdc.gov/ticks/geographic_distribution.html. Published April 2, 2020. Accessed July 7, 2020.