To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the organizational use and benefits of POCT testing in healthcare systems.
2. List personnel characteristics that should be employed when positively adapting to change.
3. Discuss tools that can be utilized when assessing the analytical performance of whole blood.
4. Describe the information and symbols that can be included in a lab report to add value and substance to the results.
Managing change is never an easy task — even less so when you are not in charge — which is where most of us find ourselves in point-of-care environments. Point-of-care testing (POCT) defines itself: Clinical laboratory testing conducted close to where patient care is provided. It is focused on real turn-around-time (TAT), from specimen collection, to measurement, and then in the hands of the caregiver. Great for the treating caregiver but it comes at a cost — a technology investment if measurement devices are dedicated to a sufficiently small group of patients and a support infrastructure such as data management systems that must work seamlessly together. Those capital costs and the related system’s complexity leads us to a need to understand how it all fits together.
Whatever the regulatory and healthcare environments are, each professional associated with POCT should have a basic understanding of their entire system and their individual responsibilities. Understanding how those responsibilities relate to the ‘big picture’ will demonstrate how critical everyone’s contribution is to overall success. Each caregiver or POCT laboratorian undoubtedly looks at this as appropriate for their own duties, training, and experience. However, the caregiver’s or laboratorian’s own view should be governed by their organization’s circumstances and needs. Key elements of the big picture always depend on combinations of the following:
· Healthcare environment:
o Primary care–standalone (e.g., community urgent care clinics, group practices) or one institutionally linked (e.g., urgent care clinics on or off campus to the sponsoring or proprietary organization)
o Secondary or tertiary care institutions where the central laboratory (CL) controls the entire POCT operation versus one where a unit/department other than the CL controls all or part of POCT operations.
-
Regulatory environment:
o CLIA’88 and state regulations set the standard.
o Standards and professional organizations Clinical and Laboratory Standards Institute (CLSI)1 and Association for Diagnostics and Laboratory Medicine (ADLM)2
o Outside the United States, other country or regional authority regulations often incorporate existing standards from standards-setting organizations such as the International Organization for Standardization (ISO).
Each team member’s application of their personal knowledge, skills, and attitude is important. Simply being a great laboratorian (or therapist or registered nurse) and applying technical knowledge and skills is not enough. In POCT, laboratorians work alongside direct care givers, sometimes in incredibly stressful and patient–critical circumstances. POCT may well not be the comfort zone.3 That is why change and its management is crucial. Note that this is not management by the ‘suits’ but by each active POCT professional. While this continuing education article is directed primarily to clinical laboratory professionals and related staff, many aspects can easily apply to other members of the POCT team.
POCT can bring much more realistic TAT for test results that are directly and immediately applied to patient care. Additionally, POCT can bring clinically better information to the bedside. The improved information may be in 1) the nature of the numeric value itself or 2) the ability to make better-informed clinical decisions because of the timeliness.
The technological advances in measurement and information management have supported many of the changes and have enabled the extension of the laboratory function to locations seen and required for both life-threatening situations or for operational efficiency (e.g., No second visit necessary for diagnosis or treatment). Clearly, this can bring about better immediate care, leading to improved clinical outcomes compared to central laboratory testing (CLT), and at the same time ensure that no patients are ‘lost’ between visit and testing and follow-up visit.4
Rather than focusing on the entire range of technical and managerial issues, this article will focus on three things: 1) Change and leading it when you are not in charge; 2) special quality control/assessment tools for POCT and their significance; and 3) specimen characteristics that affect result meaning, including how they can be readily communicated. Each of these is intertwined with the major aspects of POCT operation and management as defined by the AACC (now ADLM) Academy of Clinical Biochemistry Guideline (Academy Guideline), as well as being embodied in the CLSI Guideline mentioned above, both of which should be readily available to any professional involved in POCT.
Part I: Change management
Change is constant, or at least constantly occurring. What you thought you knew last week is different today. This requires awareness on the part of each professional in POCT. Not every change requires a new protocol or standard operating procedure (SOP), but it does require knowledge.
The management of POCT (i.e., the big picture) is outlined in the AACC (renamed ADLM) Guideline2 and encompass all major aspects that can apply in POCT. They are: 1) Interdisciplinary Committee, 2) Education,5 3) Staff and staffing, 4) PT/EQA programs, 5) Data management, 6) Selecting POCT, 7) Processes and outcomes.
‘The real picture’ reflects the AACC Guideline, but it is different. It is what each professional in POCT faces each shift. There are several aspects to one’s personal management of change, but we will focus on one and lead into two examples. The most successful change management depends on you and how you interact with other team members.
Where are you in the POCT plan? The basic expectation for each person comes from the traditional job description. It should go beyond that. An ideal job/position description that I have seen starts with the traditional job description, but adds the following in a clear, simple set of expectations: 1) Core knowledge required, 2) requisite technical skills, 3) attitude (KSA). This last item, attitude, possibly the most important, can encompass many things from internal team interactions to adhering to changing conditions cheerfully. A satisfactory attitude means that one conducts their use of knowledge and skills efficiently, pleasantly, and in accord with the reasonable expectations of the environment. Understanding these three aspects of you and the position is key to both personal and professional success. Depending on one’s own expertise or interest, discerning which of the Academy’s areas of focus is personally significant may have an impact on how you assess your own areas of activity outside the basic functions of the position.
Engage with others within the POCT Team and consider the make-up of your institution’s POCT interdisciplinary group. Considering what communities each represents may serve you well in understanding and conducting your role of managing when you are not in charge. Also see what informal systems exist to make things operate. When seeing something different than you expect, best to say, “Hey that’s a bit (or a lot) different than at my last place. Can you explain it, so I understand it better,” rather than, “I was taught” or “We did it this way at XX.” Recheck the SOP, then bring it up during coffee break.
Engage with others outside the POCT Team, especially those who POCT interacts with. Engaging with others may be a challenge for some laboratorians. This is where the Attitude from KSA comes into play. To be most effective, it is important to know something of the demands placed on each area with whom one interacts. Those accustomed to direct patient care may have different sorts of demands and perspectives. They may not, for example, understand that not all heparin is ok for use in blood collection — you are just being fussy. When you see what a registered nurse or pulmonary function technician must do, you will be much better able to understand how their ‘attitude’ affects their perspective of the ‘Lab Tech.’ Engaging with ‘others’ is likely to make you realize they are not much different than the folks in the lab — your common point is resolving issues to improve the care of the patient. And that is the key to managing change when you are not in charge.
Recent experiences with SARS-CoV-2 should be a reminder that while some change is always happening, occasionally the change is so abrupt and significant that it affects not just us but most of humanity. Being prepared suggests that having the basic technical knowledge and skills for the job isn’t enough.5 Looking outside the box and anticipating inevitable change is a requirement for survival not just success! Using the concepts described above may well be applicable to each of the topics that follow.
Part II: Assessing real system analytical performance – whole blood
The actual specimen type measured by many POCT systems is whole blood. Why then, are QC/EQA/PT materials used for validating performance made from other materials? Shouldn’t we know how well the system works on blood? An obvious example where a difference is for oxygen partial pressure (pO2). Most, if not all artificial control media cannot completely mimic whole blood for the measurement of pO2. Less obvious are the electrolytes of whole blood, especially sodium and to a lesser extent chloride.6-9
Of course, the sellers of QC materials will all say that their materials work ‘like’ blood, and they are not misleading you — all are capable of validating calibration and basic functionality. The fact is that we are accustomed to QC/EQA materials used by the central lab that are just like the actual test specimen (i.e., serum or plasma). But even whole blood–based QC materials are different than our patient test specimen. After all, they need to be stabilized to make them last for several weeks, among other modifications. One solution to this technical dilemma is duplicate analysis of the same specimen. ‘Duplicates’ were really the method of choice before the onset of commercially available materials — and it is common to use it informally.
A laboratory may choose to assess quality control by using one instrument or even two or more instruments for near-simultaneous analysis of a specimen. The utility of this approach has been thoroughly studied.10-13 An excellent guide and reference for statistical methods is published by the National Institute for Standards and Technology.14
One should expect the same measuring system to be able to repeat its performance on the same specimen. The question is how closely? Duplicate measurement of multiple specimens will give a good estimate of how closely the system routinely performs. The average difference can be calculated, as well as the random variation of the average. Once this guidance set of statistics (average of duplicates and standard deviation [SD] of duplicates) for each instrument and measurand is set, a simple monitoring plan can be established.
Similarly, two or more instruments (within or between department POCT) are assumed unlikely to have the same measurement error at the same time. Within-instrument comparison using whole blood specimen duplicates is a key to understanding system performance and evaluating complaints of performance. Both inter-instrument bias (average difference) and variance/SD can be assessed and used as an operating guideline. The duplicate measurement approach should not be used as the only method of QC. However, when used as a supplement to and in conjunction with commercial controls, it is a very useful technique for detecting errors on a particular sample (e.g., an air bubble) and for troubleshooting.
Duplicate measurements of whole blood specimens at various times during an interval of known conditions and sample stability can be used as secondary controls for detecting analyzer changes. This can reduce the need for expensive/complicated assessments using a full range of commercial controls. For duplicate values on any whole blood system:
· Check legal/institutional policy regarding use of patient blood. (If there are legal or other concerns for the use of patient blood, consider staff volunteers.)
· Set up (with statistician assistance) a protocol for duplicate testing.
· Include each measurand/analyte
· Include within and between instruments/POCT site as appropriate
· Establish performance then write a policy/practice for routine use.
The duplicate analysis of whole blood on POCT systems that measure whole blood patient specimens is essential practice and helps meet quality management guidelines, especially where it goes beyond the basic QC materials used. It can aid both within and between system assessment of performance, the latter being more significant with the common existence of multi-site measurement in the same healthcare system and the linkage of specimen reports and displays within that system.
This could be an opportunity to apply your KSA to make it happen.
Part III: Simple specimen symbols
Most laboratory information is consolidated in a laboratory information system (LIS) that links all systems and data together. However, are ‘all systems’ designed to link patient registration, wristband-scanner, the collection device itself, and the analyzers on which measurement occurs? The answers vary depending on your institution. Planning for these linkages requires awareness in the initial stages of system development, in which case the issues are relatively simple. Upfront awareness is crucial for long-term planning.
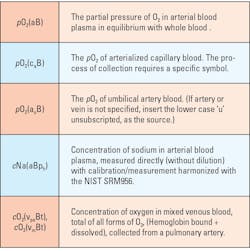
A recently proposed plan for identifying fundamental specimen characteristics can facilitate result interpretation to improve both clinical and quality management. This plan is a simple extension of a recommendation by the International Federation for Clinical Chemistry and Laboratory Medicine (IFCC). IFCC recommendations place specimen information in parenthesis following the measurand (analyte) name and identifies the anatomic source and type of specimen. The proposal simply adds symbols for unique measurement conditions that are available using modern technology (e.g., measuring sodium in the plasma of whole blood).15,16
The current system uses, for example, (aB) for arterial blood so the report would have pCO2(aB). A few additional symbols aids information system specialists since symbol sequence and position is systematic. The additional information makes it obvious if the specimen result is from a venous blood specimen pCO2(vB). If the specimen is from an arterialized capillary bed, a collection technique subject to certain unique errors, the symbol set would be for oxygen pO2(caB).
Each is linked to the specific specimen and patient, and there is no question regarding the source and its meaning for the laboratorian, caregiver, or information technology (IT) programmers who can link all from patient to collection device to the final report. An extension of this is what the sensors of the analytical systems do. Both bicarbonate and sodium are measured in a whole blood specimen but measured in the plasma. Bicarbonate could be displayed as HCO3(aBp). A similar pattern (primary anatomic source then a qualifying subscript followed by specimen type) can be used for other anatomic sources as shown in Table 1.
Measurement conditions, such as measurement in plasma (p) or plasma-water (pw) would follow the type (of specimen) symbol. Example of these currently would be blood gas systems reporting sodium, which uses ion selective electrode (ISE) technology, harmonized to agree with central laboratory systems if plasma protein/lipids levels are normal.7 Harmonized blood gas systems would/should display Sodium or Na(aBph). Most sodium values from the analyzers will agree with the central lab results. But if significantly different (remember those duplicate statistics), there may be a physiological/clinical condition, not an analytical error.
An example of the pattern/sequence for (sqTYcq) is as follows:
1) anatomic source followed by the 2) specific qualifier, (subscripted), and the 3) type of specimen then the 4) measurement condition, and its 5) qualifier (subscripted).
If you see the advantages of this approach,(i.e. Analyte Name (sqTYcq)), it is certainly something that will need some discussion, persuasion, and change management to make it work in your institution. But, given the current state of linkages between LIS/HIS and “all-in-one” data management systems, now is an optimal time.
Conclusion
Professional laboratory work in a point-of-care environment is significantly different than typical laboratory operations. By working in POCT areas, the laboratorian not only uses the technical knowledge and skills developed by education, training, and experience, but interacts with professionals who have diverse types of views of both laboratory results and of laboratorians. Coupling that with the critical care environment, which may have extremely intense and personal involvement of caregiver and patient at unpredictable times, even an experienced laboratory professional can be challenged.
When given or choosing a POCT assignment, a laboratorian should not think of it as a simple schedule or location change. It can and likely will be far more than that.
- First, you are likely to be interacting directly with the patient. Certainly, in the collection of a specimen but more than that — under acute conditions found in the emergency department/trauma, critical care units, or the operating theater.
- Second, in addition to those aspects of direct care, reflect on and prepare yourself by considering the points of part one (managing change).
- Last, if you see either or both aspects as challenges you really want, jump into it with both feet. You will not be disappointed. It will be a genuinely exciting place — a place where one can see the application of all your laboratory education, training, and experience put into effect in situations where you see what is happening to real patients, and with care given by or to people you know.
It will never be a routine day again! (Contact the author for some war stories!!)
References
- Quality Management System: A Model for Laboratory Services.
- Nichols JH, Alter D, Chen Y, et al. AACC Guidance Document on Management of Point-of-Care Testing. J Appl Lab Med. 2020;1;5(4):762-787. doi:10.1093/jalm/jfaa059.
- Jenkins, J. What can go wrong in Point of care testing. CLN. Published July/August 2023. Accessed June 28, 2024. https://www.myadlm.org/CLN/Articles/2023/JulAug/What-Can-Go-Wrong-With-Point-of-Care-Testing.
- Nichols JH. Utilizing Point-of-Care Testing to Optimize Patient Care. EJIFCC. 2021;29;32(2):140-144.
- Point of care specialist certificate program. Myadlm.org. Accessed June 28, 2024. https://myadlm.org/Education/Online-Certificate-Programs/Certificate-Programs/Point-of-Care-Specialist-Certificate-Program.
- Królicka AL, Kruczkowska A, Krajewska M, Kusztal MA. Hyponatremia in infectious diseases—A literature review. Int J Environ Res Public Health. 2020;17(15):5320. doi:10.3390/ijerph17155320.
- Villanova PA. Standardization of Sodium and Potassium Ion-Selective Systems to the Flame Photometric[ Reference Method: Approved Standard NCCLS Document C29-A2.; 2000.
- Maas A. Proposed recommendations on ion selective electrode determination of the substance concentration of sodium, potassium and ionized calcium in serum, plasma, or whole blood. In: Heiden C, Leijnse B, eds. Clinical Chemistry, An Overview. ; 1988:39-62.
- Vera MA, Sutphin A, Hansen L, El-Khoury JM. Resolving pseudohyponatremia: Validation of plasma sodium on radiometer ABL800 blood gas analyzers for immediate reflex testing. Lab Med. 2022;53(5):e105-e108. doi:10.1093/labmed/lmab114.
- Westgard JO, Moran RF, Groth T. Performance validation of blood gas instruments using three levels of controls for pH, PCO2 and PO2 - procedure and assessment by interactive computer simulation. In: Blood pH, Carbon Dioxide Oxygen and Calcium Ion (Proceedings of 5th Meeting of the IFCC Expert Panel on pH and Blood Gases). ; 1980:115-137.
- Moran RF. Assessment of quality control of blood gas/pH analyzer performance. Respir Care. 1981;26:538-546.
- Burnett RW, Maas AH, Moran RF. Quality control in blood pH and gas analysis by use of a tonometered bicarbonate solution and duplicate blood analysis. Clin Chem. 1981;27(10):1761-1764. doi:10.1093/clinchem/27.10.1761.
- Elser R, Hess D, Moran RF. Assessment of the agreement between duplicate whole blood measurements of blood gases and pH on independently calibrated analyzers. Methodology and Clinical Applications of Electrochemical and Fiber Optic Sensors. 1990;11.
- National Institute of Standards and Technology (NIST), SEMATECH. e-Handbook of Statistical Methods. Available at: https://www.itl.nist.gov/div898/handbook/. Accessed June 28, 2024.
- Moran RF. POC Testing and Reporting of Sodium, and Other Small Molecules Need Modified IFCC Source/Type Designations to Improve Operational Efficacy and for Clinically Accurate, Unambiguous Reporting from LIMS and HIS. EJIFCC. 2023;21;34(4):271-275.
- Moran RF. Overcoming data management challenges: The biggest challenge of all is….us. Medical Laboratory Observer. Published January 29, 2024. Accessed June 28, 2024. https://www.mlo-online.com/information-technology/data-management/article/53081209/overcoming-data-management-challenges-the-biggest-challenge-of-all-isus.
To take the test online go HERE. For more information, visit the Continuing Education tab.