Hepatitis C virus (HCV) is a major health concern both in the United States and abroad. Chronic infection with HCV is a leading cause of cirrhosis and, due to its association with hepatocellular carcinoma, is the most common indication for liver transplant in the United States.1
The prevalence of HCV infection in the United States is estimated to be five to seven million cases, or 1.6 percent to 1.8 percent of the total population.2 Due to a confluence of risk factors, including contaminated blood transfusions and expanded use of illicit intravenous drugs, about three-fourths of cases of chronic HCV infection in the United States belong to a single cohort of people born between 1945 and 1965.3 Thus, the U.S. Centers for Disease Control and Prevention (CDC) and United States Preventive Services Task Force recommend routine screening in that population.4-6
HCV Ab enzyme-linked immunosorbent assay
Historically, screening for HCV infection has been performed by enzyme-linked immunosorbent assay (ELISA) for antibodies against viral proteins. Anti-HCV antibodies can be detected in infected individuals as early as four to ten weeks after exposure.7 Third generation ELISAs, the latest iteration available, achieve superior sensitivity over preceding versions by targeting multiple viral antigens, including the core and nonstructural (NS) proteins N3, NS4, and NS5.8,9 Though ELISAs have high specificity when testing at-risk populations,10 they have a low positive predictive value when used for wider screening. Thus, a positive result must be confirmed by a more specific test. Other limitations of ELISA include the potential for a false-negative result in patients with deficiencies in humoral immunity—e.g., patients who are immunocompromised, who are on hemodialysis, or who are the recipients of organ transplants.
Rapid testing
Point-of-care tests (POCTs) for HCV infection have been developed to provide a presumptive diagnosis quickly and without a clinical laboratory. Rapid tests for anti-HCV antibody target the same viral antigens as do third-generation ELISAs, but can be used on fingerstick capillary whole blood as well as venipuncture-collected whole blood. Nonetheless, on evaluation by the CDC in various clinical settings, rapid POCTs were shown to perform as well as ELISA.11- 13
To date, only one POCT has been approved by the FDA for the detection of anti-HCV antibody in at-risk individuals or those with suspected hepatitis.14 The approved POCT has been granted a Clinical Laboratory Improvements Amendments waiver permitting its use in physician offices, clinics, and emergency departments. Because POCTs are more costly than traditional ELISAs and cannot be batched, their utility in high volume settings is likely limited. Importantly, POCTs have not been approved for general screening.
Recombinant immunoblot assays
Recombinant immunoblot assays (RIBAs) are highly specific tests to confirm the presence of anti-HCV antibody after a positive result on ELISA. Because RIBAs require serum to react with at least two viral antigens to be called positive, patients with early infection may receive an indeterminate result if they have not yet mounted a full immune response to the virus. RIBAs are costly, slow, and somewhat impractical, in that a positive result must be followed by an assay for HCV RNA before a diagnosis of active infection can be made. Thus, RIBAs are no longer recommended for clinical use.15
Molecular testing
Nucleic acid testing (NAT) detects HCV RNA in the serum or plasma of actively infected individuals, most commonly by real-time polymerase chain reaction. In 2013, the CDC updated its guidelines to include NAT as the recommended confirmatory test for HCV infection following a positive screening ELISA.6 NAT is extremely sensitive—able to detect viral loads as low as 10-15 IU/mL16 —and highly specific, regardless of HCV genotype. Because NAT does not require circulating anti-HCV antibodies for a positive result, it may be used to assess for acute HCV infection as early as one to three weeks after exposure or in an immunocompromised host. Thus, though no NAT has been approved by regulatory agencies for the diagnosis of HCV infection, clinicians will commonly request NAT when there is suspicion for acute HCV infection despite a negative assay for anti-HCV antibody.
Once active HCV infection is confirmed, determination of the viral genotype can guide treatment and inform prognosis. The only FDA-approved and commercially available genotype assay uses real-time PCR to detect portions of the 5’untranslated region and the core/NS5B amplicon.17 In a post-market evaluation of that assay, about 2 percent of tests yielded an indeterminate result; 17 of 19 of these indeterminate cases were subsequently identified as genotype 3a.18
HCV core antigen testing
An assay for direct detection of HCV antigen from venous blood is currently available in Europe. Direct antigen testing (DAT) quantitates a viral load based on relative chemiluminescence from a bead-coated monoclonal antibody to the HCV core antigen.19 As with NAT, a positive test indicates active HCV infection and does not rely on the body’s generation of anti-HCV antibodies. Though DAT does not perform as well as NAT in the setting of low viral loads, samples submitted for DAT require less processing than do molecular assays and therefore may appeal for use in clinical laboratories. A summary of clinical diagnostics for HCV is presented in Table 1.
Table 1. Laboratory diagnostics for HCV infection.
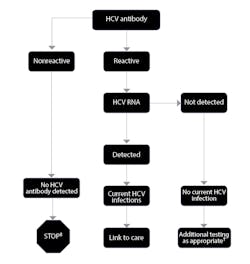
The CDC has proposed an algorithm to guide the use of the many available diagnostics for HCV (Figure 1). Given the new recommendations for screening patients born between 1945 and 1965—ie, “baby boomers”—clinical laboratories should expect to see higher volumes of testing. By understanding the role for each test, laboratories and their staff can plan accordingly.
Figure 1. CDC algorithm for diagnosis of current HCV infection
References
- Kim WR, Smith JM, Skeans MA, Schladt DP, Schnitzler MA, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 Annual Data Report: liver. Am J Transplant. 2014 Jan;14 Suppl 1:69-96.
- Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011 Sep;31(8):1090-1101.
- Ansaldi F, Orsi A, Sticchi L, Bruzzone B, Icardi G. Hepatitis C virus in the new era: perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J Gastroenterol. 2014 Aug 7;20(29):9633-9652.
- Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N, Alter M, Yartel A, Ward JW. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012 Aug 17;61(RR-4):1-32.
- Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3;159(5):349-357.
- Centers for Disease Control and Prevention (CDC). Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013 May 10;62(18):362-5.
- Kamili S, Drobeniuc J, Araujo AC, Hayden TM. Laboratory diagnostics for hepatitis C virus infection. Clin Infect Dis. 2012 Jul;55 Suppl 1:S43-8.
- Alter HJ. New kit on the block: evaluation of second-generation assays for detection of antibody to the hepatitis C virus. Hepatology. 1992;15:350-353.
- Barrera JM, Francis B, Ercilla G, et al. Improved detection of anti-HCV in post-transfusion hepatitis by a third-generation ELISA. Vox Sang 1995;68:15-8.
- Colin C, Lanoir D, Touzet S, Meyaud-Kraemer L, Bailly F, Trepo C. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat 2001;8:87-95.
- Smith BD, Drobeniuc J, Jewett A, et al. Evaluation of three rapid screening assays for detection of antibodies to hepatitis C virus. J Infect Dis. 2011;204:825-31.
- Smith BD, Teshale E, Jewett A, et al. Performance of premarket rapid hepatitis C virus antibody assays in 4 national human immunodeficiency virus behavioral surveillance system sites. Clin Infect Dis. 2011;53:780-6.
- Shivkumar S, Peeling R, Jafari Y, Joseph L, Pant Pai N. Accuracy of rapid and point-of-care screening tests for hepatitis C: a systematic review and meta-analysis. Ann Intern Med. 2012;157:558–566.
- Food and Drug Administration. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfClia/detail.cfm?id=5088620&NoClia=1&pagenum=500. Accessed February 26, 2015.
- Cobb B, Heilek G, Vilchez RA. Molecular diagnostics in the management of chronic hepatitis C: key considerations in the era of new antiviral therapies. BMC Infect Dis. 2014;14 Suppl 5:S8.
- Cobb B, Pockros PJ, Vilchez RA, Vierling JM. HCV RNA viral load assessments in the era of direct-acting antivirals. Am J Gastroenterol. 2013 Apr;108(4):471-475.
- Mallory MA, Lucic DX, Sears MT, Cloherty GA, Hillyard DR. Evaluation of the Abbott realtime HCV genotype II RUO (GT II) assay with reference to 5’UTR, core and NS5B sequencing. J Clin Virol. 2014 May; 60(1):22-26.
- González V, Gomes-Fernandes M, Bascuñana E, Casanovas S, Saludes V, Jordana-Lluch E, Matas L, Ausina V, Martró E. Accuracy of a commercially available assay for HCV genotyping and subtyping in the clinical practice. J Clin Virol. 2013 Sep; 58(1):249-253.
- Muerhoff AS, Jiang L, Shah DO, et al. Detection of HCV core antigen in human serum and plasma with an automated chemiluminescent immunoassay. Transfusion 2002;42:349-356.