Intra-abdominal infections are often implicated as a cause of sepsis. The resultant action of inflammatory cytokines and/or the body’s reaction to sepsis may lead to acute renal failure, defined as a sudden, sharp decline in renal function. The purpose of this paper is to describe the diagnosis, pathophysiology, and treatment of acute renal failure secondary to sepsis. The course and progress of the disease is illustrated using the case study of an HIV-positive male. The patient was admitted to the emergency department complaining of nausea, diarrhea and lower flank pain. Physical examination revealed a questionable right abscess and lower flank pain consistent with appendicitis, which was confirmed by appendectomy surgery shortly after admission. Lab results upon admission were consistent with infection and acute renal failure.
Acute renal failure
Acute renal failure is a sudden, sharp decline in renal function caused by toxins or conditions resulting in tissue hypoxia. Acute renal failure is subdivided into three classes based on the origin: pre-renal failure, primary renal failure, and post-renal failure.1 Pre-renal failure is caused by a defect in the blood supply to the kidneys, resulting in tissue hypoxia. Primary renal failure is caused by a defect with the kidneys themselves involving the glomeruli or tubules. Post-renal failure is caused by a defect in the portion of the urinary tract after it exits in the kidneys. Of these three classes, pre-renal failure is the most common. The symptoms of acute renal failure are oliguria and anuria, edema, hypotension, congestive heart failure, and increased levels of blood urea nitrogen (BUN) and creatinine. The condition may be followed by recovery or progression to chronic renal failure if severe.1
Pathophysiology
Acute renal failure secondary to intra-abdominal infection is primarily mediated through the action of inflammatory cytokines. Intra-abdominal infection is caused when bacteria are introduced into the peritoneal space through bowel perforation or introduced externally via trauma. In our case study, the intra-abdominal infection was secondary to appendicitis. A small portion of impacted fecal matter blocked the patient’s appendix, trapping fecal bacteria and resulting in inflammation and necrosis. The necrotic tissue of the wall weakened and allowed bacteria to seed the peritoneal cavity, resulting in inflammation of the peritoneum, formation of abdominal abscess, and sepsis. Sepsis is a severe inflammatory reaction to an infection. The inflammation of the peritoneum, through the action of inflammatory cytokines, caused bowel irritation, leading to episodes of diarrhea.2 Sepsis results in the production of inflammatory cytokines such as tumor necrosis factor, interferon, and interleukin-1. These cytokines can induce nitrous oxide production by the kidneys, causing systemic vasodilation and resulting renal vasoconstriction.3 The systemic vasodilation can lead to decreased blood flow to the kidneys, causing ischemia and renal damage. The diarrhea and vasodilation both play a role as causal agents of acute renal failure.
In the case of prolonged diarrhea, severe volume depletion of the extracellular fluid can occur, producing a condition called hypovolemia. In hypovolemia, there is a decreased blood supply to the kidneys. In response, there is an increased release of antidiuretic hormone (ADH) from the pituitary gland. The increased levels of ADH result in elevated blood urea nitrogen (BUN) and creatinine levels and decreased urine volume. Typically the ratio of BUN to creatinine is high. Increased activity of angiotensin II, adrenergic activity, and aldosterone results in increased proximal and distal tubular reabsorption. Renal failure results when the kidney’s compensatory mechanism is inadequate, resulting in a drastic reduction in the glomerular filtration rate (GFR).4 Prolonged volume depletion can lead to ischemia. Irreversible damage may take place and with progression to chronic end stage renal failure.
In the case of sepsis, vasodilation leads to poor renal circulation, ischemia and resistance to vasoconstrictors, norepinephrine and angiotensin II.5 This resistance heightens the effect of vasodilation due to nitrous oxide, leading to ischemic nephron damage. 4
Clinical presentation, diagnosis, and treatment
Pre-renal azotemia is the condition characterized by increased levels of BUN as can occur with volume depletion. As damage to the nephrons continues, pre-renal azotemia, a normally reversible condition, can progress to chronic renal failure. Clinical signs of pre-renal azotemia include edema, thirst, oliguria, symptoms of heart failure, dry mucous membranes, orthostatic hypotension, and rapid pulse.6 Urinalysis is usually normal and urinary indices are normal. The BUN: creatinine ratio is often elevated. A BUN: creatinine ratio between 10:1 and 20:1 is considered normal. A BUN: creatinine ratio 6 As kidney damage continues, pre-renal azotemia can progress to chronic end stage renal failure. Clinical signs of chronic renal failure include proteinuria, cellular casts, and low urine specific gravity.
The laboratory plays a key role in the diagnosis of acute and chronic renal failure by performing serum chemistries and urinalysis testing. The basic metabolic panel in addition to urine dipstick and microscopic exam allowed initial diagnosis and monitoring of disease progression. The Microbiology department also plays a role in assessing the patient’s HIV seropositive status and ruling out other possible causes of the diarrhea. Hematology results are instrumental in identifying anemia secondary to renal failure. The WBC differential count is also key in identifying the presence of infection.
Patients usually receive dialysis which alleviates dangerously elevated potassium levels and electrolyte disturbances, and lowers BUN and creatinine levels. Patients are prescribed antimicrobial agents to combat infection.
Case study
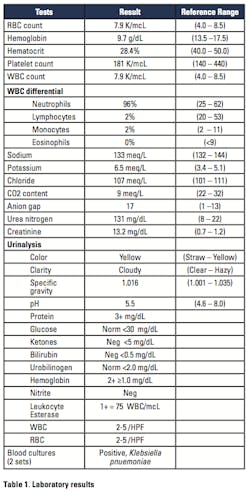
An HIV-positive adult male presented to the Emergency Department complaining of nausea, diarrhea lasting for the past eleven days with 10 episodes per day, and lower flank pain. The patient history included a 20-to-30 pound weight loss, fatigue, night sweats, and chest pains in conjunction with diarrheal episodes. Upon admission, the patient’s temperature was 38.7 degrees Celsius. Physical examination revealed a right pelvic abscess and lower flank pain consistent with appendicitis. Initial laboratory results reflected infection, metabolic acidosis, hyperkalemia, and acute renal failure. The patient’s blood culture was positive for Klebsiella pnuemoniae (Table 1).
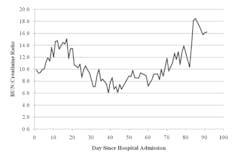
Two days after admittance, the patient had an appendectomy. Surgical findings were severe acute serositis, recurrent necrosis, fecalith, and fibrosed appendix containing an exudate. The patient received dialysis treatment shortly after admittance to address high BUN and creatinine levels, hyperkalemia, and metabolic acidosis. Hospitalization was prolonged due to complications—respiratory failure, disseminated intravascular coagulation, myocardial infarction, and peripheral edema. The patient’s BUN: creatinine ratios over the course of his hospital stay are illustrated in Figure 1. The patient was also administered highly active retroviral therapy (HAART) drugs and antimicrobial agents. However, normal renal function was not recovered for an extended period of time.
Figure 1. Blood urea nitrogen: creatinine ratio vs. day since hospital admission
Acute renal failure is a sudden loss in renal function due to pre-renal, renal, or post-renal disturbances. Sepsis and inflammation are important causal agents of pre-renal kidney failure, which may progress to chronic end stage renal disease if conditions are left untreated. The HIV seropositive population is at higher risk for acute renal failure compared to the immuncompetent population due to the increased likelihood of severe infection. Adherence to HAART and seeking medical attention early in cases of illness will limit the possibility of severe renal damage.7
Floyd Josephat, EdD, MT(ASCP), is Assistant Professor in the Medical Laboratory Science Department at Armstrong Atlantic State University in Savannah, GA. He has more than 20 years of laboratory experience. Denene Lofland, PhD, (middle) earned her degree in Pathology from Virginia Commonwealth University School of Medicine. She is currently the Interim Department Head of Medical Laboratory Science at Armstrong Atlantic State University. Katrina Jackson BS, MT(ASCP), graduated from Armstrong Atlantic State University in 2010. She is an ASCP-certified Medical Laboratory Scientist currently working in the Transfusion Service department of Grady Memorial Hospital in Atlanta.
References
- Bishop ML, Fody EP, Schoeff LE. Clinical Chemistry: Techniques, Principles, Correlations. 2010. Baltimore, MD: Lippincott Williams & Wilkins, a Wolters Kluwer business.
- Binder H. Mechanisms of diarrhea in inflammatory bowel diseases. Annals of the New York Academy of Sciences. 2009;1165285-293. doi:10.1111/j.1749-6632.2009.04039.x.
- Majumdar A. Sepsis-induced acute kidney injury. Indian Journal of Critical Care Medicine. 2010;14(1):14-21. Retrieved from Academic Search Complete database.
- Blantz RC. Pathophysiology of pre-renal azotemia. Kidney International Supplement. 1998;(53):512-523. Retrieved from Academic Search Complete database.
- Schor N. Acute renal failure and the sepsis syndrome. Kidney International. 2002;61(2):764. Retrieved from Academic Search Complete database.
- Dwinnell BG, Anderson RJ. Diagnostic evaluation of the patient with acute renal failure. Retrieved from www.kidneyatlas.org.
- Bova R, Meagher A. Appendicitis in HIV-positive patients. Australian & New Zealand Journal of Surgery. 1998;68(5):337. Retrieved from Academic Search Complete database.