Diabetes is a significant public health issue in the United States. According to the U.S. Centers for Disease Control and Prevention (CDC), 34.2 million people – or 10.5 percent of the U.S. population – have diabetes, which is the 7th leading cause of death in the United States.1
Clinicians typically diagnose diabetes using either fasting plasma glucose (FPG) value, 2-h plasma glucose (2-h PG) during a 75-g oral glucose tolerance test (OGTT), or Hemoglobin A1c (A1c or HbA1c).
When using an A1c method, laboratorians should use a method that has been certified by the National Glycohemoglobin Standardization Program (NGSP) and standardized or traceable to the Diabetes Control and Complications Trial (DCCT) reference assay.2 This article examines some of the methods used to measure A1c that meet both of those criteria.
High-Performance Liquid Chromatography (HPLC)
HPLC is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. Pressurized liquid solvent containing the sample mixture passes through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column.
The components of the sample mixture separate from each other due to their different degrees of interaction with the adsorbent particles. There are various types of HPLC such as ion exchange, reverse phase, size exclusion, partition, normal phase and affinity chromatography.3
Both ion-exchange and affinity chromatography are used to measure A1c. Although both fall in the category of HPLC, they use different separation theories. In ion exchange-HPLC, separation is based on charge, while in affinity chromatography, separation is based on structure.
Cation-Exchange HPLC
In cation-exchange HPLC, a type of ion-exchange-HPLC, separation of hemoglobin molecules is based on charge, and hemoglobin molecules are positively charged.
Red blood cells are lysed and passed through a negatively charged resin packed in a column. Positively charged hemoglobin molecules interact with the negatively charged resin, so the negatively charged molecules move at a faster rate. The bound hemoglobins are released by varying solvent conditions injected into the column (e.g., increasing the ion effect of the solvent system by increasing the salt concentration of the solution, increasing the column temperature, changing the pH of the solvent, etc.).3
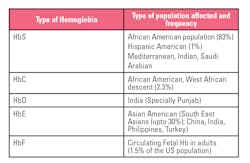
A chromatogram shows the eluted components in the form of quantifiable peaks. The advantage of using cation-exchange HPLC is that it not only provides an A1c value but also provides presumptive identification of commonly occurring hemoglobin variant traits that can potentially interfere with A1c results.
Table 1 provides a quick snapshot of these commonly occurring variants.4
In this method, particular attention is given to the quality, type and size of resin used in packing a column (non-porous resin materials have been shown to be an efficient tool in A1c analysis),5 and in consistency measures adopted in the column packing process. The quality of resolution of peaks in the chromatogram is equally important in this method. A well resolved A1c peak yields an accurate result. Partiular attention is also given the the simplicity of the A1c result. Some manufacturers use a direct measure of area under the peak, eliminating the need for complex calculations.
Boronate affinity chromatography
As mentioned earlier, this technique is an HPLC method that separates each component in a mixture based on structure. The high specificity of boronate makes it suitable for the separation of compounds, like glycated hemoglobins, that have a cis-diol in their molecular structure. The basic interaction for boronate chromatography is esterification between boronate ligands and cis-diols configuration formed by stable glucose attachments to Hb. As the glycated and non-glycated products elute through a column, the hemoglobin interacts with boronic acid giving 2 peaks: glycated (GHb) and non-glycated hemoglobin.6,7 This method is less prone to interferences with variants; however, it lacks the ability to presumptively identify variant hemoglobins. Interference with A1c results in the presence of HbF above 15 percent is seen in this method.8 The method does not directly measure A1c but calculates A1c from the glycated fraction.
Capillary electrophoresis (CE)
Capillary Electrophoresis (CE) separates Hb molecules based on charge and mass. The charged molecules (in this context, the hemoglobins) are resolved by their electrophoretic mobility. The technique uses capillary tubes through which high-voltage electricity is used to generate an electric field that facilitates the migration of hemoglobins from anode to cathode.
The separation of the hemoglobin species occurs in decreasing order of their charge-to-mass ratio, with the positively charged hemoglobins eluting first. If there are two or more molecules that have the same charge, the system will further resolve these by size, and the large molecules will elute first. Positively charged molecules are followed by neutral species and, finally, by negatively charged Hb species.
Capillary electrophoresis is a more recent technology when it comes to HbA1c detection, compared with HPLC and immunoassay. Just as in cation-exchange HPLC, this method allows for presumptive identification of hemoglobin variants that can be seen on an electropherogram. The most common heterozygous Hb variants (HbS, HbC, HbD, and HbE) do not interfere with the method.9
However, analyzers using this method may need more maintenance because of the number of small-diameter capillaries necessary to carry out testing. High-volume laboratories will benefit from such analyzers only if multiple analyzers can be connected to a track-line.
Immunoassays
Immunoassays are bioanalytical methods in which the quantitation of the analyte depends on the reaction of an antigen with an antibody where the antigen is usually the analyte tested for. The competitive (inhibition) immunoassay is based on a competitive binding reaction between a fixed amount of a labeled form of an analyte and a variable amount of unlabeled sample analyte for a limited amount of binding sites on a highly specific anti-analyte antibody. When these immunoanalytical reagents are mixed and incubated, the analyte is bound to the antibody forming an immune complex.
This complex is separated from the unbound reagent fraction by a physical or chemical separation technique. Analysis is achieved by measuring the label activity (e.g. radiation, fluorescence, or enzyme) in either of the bound or free fraction. A standard curve, which represents the measured signal as a function of the concentration of the unlabeled analyte in the sample, is constructed. Unknown analyte concentration is determined from this calibration curve.10
In the context of A1c, immunoassays use antibodies that target N-terminal glycated amino acids on the ß chain to quantify A1c. The quality of an immunoassay very much depends on the specificity of the antibody to the first few amino acids on ß chain of HbA. Point mutations for HbS and HbC occur at ß6 and care must be taken to choose a method where the antibody epitope does not span that area. There are other uncommonly occurring variants that span this epitope. Further, the point mutations for HbE and HbD (Los Angeles) occur at ß26 and ß121 respectively.
Immunoassay analyzers are known for their speed of analysis and satisfy the needs of laboratories running a high volume of A1c samples. For laboratories running a menu of analytes, the ability to consolidate assays on one single analyzer becomes an important decision-maker in choosing an analyzer. The biggest argument against selecting an immunoassay method is the inability of the method to presumptively identify variant hemoglobins. The A1c result is merely a number.1 Another important consideration is the physical space constraints of a laboratory in accommodating an analyzer with a large footprint. Laboratories must be aware of the trade-offs in selecting an immunoassay analyzer for A1c.
Enzymatic methods
In this method, whole blood samples are subject to breakdown of protein into amino acids and small peptides (proteolytic digestion). HbA1c is a glycated hemoglobin in which glucose is bound specifically to the N-terminal valine of the Hbβ chain. Proteolytic digestion releases the glycated N-terminal valine from the Hbβ chains. Glycated valine serves as a substrate for a specific enzyme that cleaves the N-terminal valine and produces hydrogen peroxide that is subjected to a chromogenic reaction. The signal produced is directly proportional to the amount of A1c in the sample. Just as in immunoassay, this method provides only an A1c value and no additional information about the presence of hemoglobin variants or labile and carbamylated Hb.9
This overview suggests that a number of factors are involved in choosing the right analyzer for a laboratory’s needs. In addition to the accuracy and precision of the test results, other common considerations include sample throughput, workflow efficiencies, frequency of instrument maintenance, sample workload, environmentally friendly disposal of reagents, and compatibility with track-line automation.
Variant interferences play an equally important role. In the United States, where the population is ethnically and racially diverse, the ability for a method to presumptively identify commonly occurring hemoglobin traits provides an additional level of certainty in the result.
In conclusion, every laboratory has different needs. When choosing an analyzer, it is important to meet the economic needs of the laboratory without compromising on the quality of an A1c result, which is becoming an ever-more-important tool for diagnosing and monitoring diabetes.
References
- Diabetes Data and Statistics. CDC website. https://www.cdc.gov/diabetes/data/index.html
- Standards of Medical Care in Diabetes. Diabetes Care. 2020; 43 (Supplement 1).
- High Performance Liquid Chromatography. Wikipedia website. https://en.wikipedia.org/wiki/High-performance_liquid_chromatography
- Sivaraman P, Patel N, HPLC: its continuing role in diabetes monitoring. Medical Laboratory Observer. June 2015.
- Nakatani S, Kitamura T, Yamasaki Y, Kato Y. Separation of glycated hemoglobin A1c by high performance Liquid Chromatography on a Non-Porous Exchanger. Chromatographia. 1991;31(9/10):505-506.
- Liu X, Scouten W. Boronate Affinity Chromatography. In: Bailon P et al. Affinity Chromatography. Humana Press; 2000: 119-128.
- Chauhan N. Laboratory Diagnosis of HbA1c: A Review. Journal of Nanomedicine Research. 2017;5(4).
- HbA1c Assay Interferences. NGSP website. http://www.ngsp.org/interf.asp
- Rhea J, Molinaro R. Pathology consultation on HbA1c methods and interferences. Am J Clin Pathol. 2014;141(1): 5–16.
- Kellner R, Mermet J, Otto M, Widmer H. Analytical Chemistry. New York: Wiley-VCH; 1998: 405–429.
- Sacks D. Hemoglobin Variants and Hemoglobin A1c Analysis: Problem Solved? Clinical Chemistry. 2003;49(8): 1245-1247
- Hanley T, Signorelli, D. Considerations in Choosing Hemoglobin A1c Methods. Clinical Laboratory News. March 2015.