Addressing STI challenges: A diagnostic update on the current landscape and future strategies
The relentless rise in curable bacterial sexually transmitted infections (STIs) across the United States is an urgent public health crisis.1 In the latest report from the Centers for Disease Control and Prevention (CDC) in 2021, there was a staggering number of cases, including over 1.6 million new cases of Chlamydia trachomatis, 700,000 for Neisseria gonorrhoeae, and 170,000 for syphilis. This represents a continued increase in C. trachomatis and N. gonorrhoeae infections, and a notable 32% increase in syphilis cases compared to the preceding year.1,2
In addition to the increases in reported STIs, antimicrobial resistance is also growing.2 Nearly half of N. gonorrhoeae infections were antimicrobial resistant in 2021, signaling a critical challenge.2 Moreover, the emergence of resistance in infections like Mycoplasma genitalium has raised additional concerns.3,4 Although not recommended for routinely screening, M. genitalium is prevalent in 15% to 40% of cases of persistent or recurrent urethritis.3,4 Estimates point to around three million prevalent cases of M. genitalium in the United States.5
Effective management of the STI epidemic will require the adoption of innovative and accurate diagnostic tools that are practical, rapid, and available at or near the point of care. Improved diagnostics will not only expedite STI detection and treatment, but also detect drug resistance in real time to promptly guide therapy and foster antimicrobial stewardship.
Diagnostic landscape
Chlamydia trachomatis and Neisseria gonorrhoeae
Molecular technology has significantly transformed the field of STI diagnostics. Today, rapid point-of-care assays based on nucleic acid amplification tests (NAATs) are available for the detection of C. trachomatis and N. gonorrhoeae and increasingly recognized as gold standard assays due to their performance profiles, with sensitivities and specificities exceeding 95%.6 A noteworthy development lies in newer molecular diagnostic technologies, which are designed as combination assays, allowing for the detection of multiple pathogens to potentially enhance case management.7
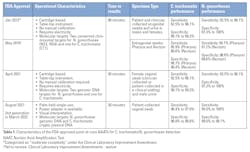
Three point-of-care Food and Drug Administration (FDA)–cleared devices are currently available for the simultaneous detection of C. trachomatis and N. gonorrhoeae. These polymerase chain reaction (PCR)-based tests amplify target pathogen genomic DNA to provide results within 30 to 90 minutes, without requiring user calibration. The characteristics and performance of the FDA-approved molecular point-of-care C. trachomatis/N. gonorrhoeae tests are highlighted in Table 1. Some have received a Clinical Laboratory Improvement Amendments (CLIA) waiver.8-11Mycoplasma genitalium
Although M. genitalium screening is not routine in the United States, CDC guidelines now recommend for testing in cases of recurrent or persistent urethritis or cervicitis.3,4 Rapid point-of-care M. genitalium tests are not available in the United States, but two automated high-throughput laboratory-based NAATs have FDA clearance for detecting M. genitalium in male and female specimens, including urine and self-collected vaginal swabs.
The first assay uses transcription-mediated amplification and hybridization protection assay technologies to detect the 16s rRNA of M. genitalium.12 The second, recently FDA-cleared in 2022, is a reverse transcriptase PCR, four-in-one multiplex test to simultaneously detect C. trachomatis, N. gonorrhoeae, Trichomonas vaginalis, and M. genitalium DNA.13
Resistance-guided therapy
N. gonorrhoeae and M. genitalium are of concern due to their increasing resistance to antibiotics.3 Both STIs are on the CDC’s resistant threat watchlist.14 Over time, N. gonorrhoeae has quickly developed resistance to nearly all antibiotics used in its treatment. The CDC’s Gonococcal Isolate Surveillance Project monitors antimicrobial susceptibility in about 3% of urethral isolates in the United States and of the isolates sampled in 2021, around half showed high minimum inhibitory concentrations (MIC) to at least one antibiotic.3,15 However, it is worth noting that all cases still respond to injectable ceftriaxone, an antibiotic from the cephalosporin class and the current recommended treatment for uncomplicated N. gonorrhoeae infection.2
While there is yet to be a standardized laboratory criterion for cephalosporin resistance in N. gonorrhoeae, reduced susceptibility is generally considered when MICs exceed 0.5 µg/ml. Notably, there have been identified cases of isolates with decreased susceptibility to ceftriaxone (MIC: 1.5-4.0 µg/ml) in East Asia and Europe.3,15
Laboratorians and healthcare providers are urged to stay vigilant and report instances of cephalosporin resistance or treatment failure to the CDC. Clinical laboratories should also send specimens to the CDC for further analysis. When N. gonorrhoeae treatment failure is suspected, culture and antimicrobial susceptibility testing are still the standard assessment. Agar dilution is the preferred method for susceptibility testing, although qualitative MIC determination using the Etest is acceptable.3,15
To improve treatment decisions and slow the spread of antimicrobial resistance, there is an urgent need for molecular assays that can identify genetic mutations that predict antibiotic resistance in N. gonorrhoeae in real time. Utilizing a resistance-guided therapy approach for N. gonorrhoeae may facilitate the use of alternative antibiotics such as ciprofloxacin in susceptible strains, which may decrease the selective pressure for resistance to cephalosporins.16
While there are various mutations that are associated with ciprofloxacin resistance in N. gonorrhoeae, it is the absence of a single mutation at the serine 91 codon of the gyrase A (gyrA) gene that confers susceptibility to ciprofloxacin.17 As such, real-time PCR-based molecular assays have been developed to predict gonococcal susceptibility to ciprofloxacin, which have been implemented in clinical practice.17,18 In a multicenter clinical trial assessing resistance-guided therapy through gyrA genotyping, a treatment efficacy rate of 100% (1-sided 95% confidence interval [CI], 97.5%–100%) for ciprofloxacin was observed when gyrA genotyping predicted susceptibility to ciprofloxacin.16 In the latest STI Treatment Guidelines, the CDC recommends the use of gyrA testing to detect ciprofloxacin susceptibility and to treat with a single oral dose of ciprofloxacin 500 mg among persons with predicted susceptible strains.15
Multiple mutations in penA and non-penA genes have been identified as potential markers of N. gonorrhoeae resistance to cephalosporins. PenA mosaicism has been linked to ceftriaxone resistance, but not all strains conform to this pattern. Non-penA mutations, including ponA (L421P), penB (G120/A121), and mtrR (−35delA), are also crucial markers, particularly among non-mosaic strains. Several assays are under development, each with varying sensitivity and specificity.15 In a study from Canada, an assay that targeted three or more mutations for detection had 98.3% sensitivity but low specificity (66.7%).19 The most effective mutation combination is yet to be determined. Geographic variation and heterogeneity in molecular markers introduce further complexity, with some mutations being more prevalent in certain regions. Precision could be improved by tailoring assays to the local epidemiology and by increasing genomic surveillance. Future work should evaluate assays in diverse scenarios.18
In 2016, a study found high prevalence of macrolide-resistant M. genitalium (51% from females and 42% from males).20 Increasing resistance in M. genitalium has led to recommendations that a positive M. genitalium result should be routinely followed by a test for resistance.3,21 Currently, laboratory sequencing methods like Sanger sequencing and pyrosequencing can identify single base macrolide-resistance-mediating mutations at positions 2058/2059 of the M. genitalium 23S rRNA gene.21,22 Rapid diagnostic tests that detect M. genitalium resistant mutations to macrolide antibiotics are already available in Europe. Their evaluation is still ongoing in the United States and will represent a pivotal step toward effective management of resistance in the context of M. genitalium infections.3,4Syphilis
The bedrock of syphilis diagnosis still lies in serologic testing.23 Both treponemal tests (detecting IgG or IgM antibodies against Treponema pallidum) and nontreponemal tests (identifying non-specific antigens, such as cardiolipin produced in response to an active infection) are essential for screening and confirming diagnosis of an active infection.23 Gaps in completing the testing-to-treatment cascade have contributed to the surge in syphilis cases in the United States.1 Rapid point-of-care serologic treponemal tests may bridge these gaps.
Commercially available today are two FDA-cleared rapid point-of-care treponemal tests with good performance (see test characteristics in Table 2).24-26 The first, receiving CLIA waived status in 2014, employs lateral flow to detect treponemal antibodies by binding to recombinant T. pallidum proteins to generate a visual band that indicates a reactive result.25,26 The second test, recently achieving its CLIA waiver in 2023, uses a proprietary dual immune-chromatographic path platform to simultaneously detect antibodies against HIV types 1 and 2 and T. pallidum (for syphilis). It employs a microreader for results.26 Treponemal antibodies in an infected sample bind the recombinant T. pallidum antigens integrated into both assays (Table 2).Diagnostic stewardship
Antimicrobial stewardship is a strategy against antimicrobial resistance and a priority in mitigating resistant sexually transmitted pathogens as well as other infectious diseases. Stewardship focuses on preventing antibiotic overuse, misuse, and abuse.36 Delayed STI results resulting in empiric therapy has been a major contributor to antimicrobial resistance in STIs.37 As such, accurate rapid diagnostics can overcome obstacles in timely diagnosis and counteract the rise of antimicrobial resistance. Furthermore, rapid diagnostics that afford real-time, resistance-guided therapy could also streamline antimicrobial use in STIs.
Meanwhile, the concept of "diagnostic stewardship" is worth highlighting. Recently introduced in the domain of antimicrobial stewardship, diagnostic stewardship aims to ensure that the right test is administered to the right patient at the right time.38 As modern molecular diagnostic technologies advance into multiple target combination assays, cautiousness is advised, to prevent overdiagnosis and overtreatment, which could undermine the primary purpose of antimicrobial stewardship.
Conclusion
The STI crisis and growing antimicrobial resistance underscore the need for accurate, clinically actionable diagnostics. Advancements in molecular diagnostics offer rapid STI testing, while serologic tests remain pivotal for syphilis. Addressing resistance requires ongoing surveillance and the implementation of assays for detecting resistance mutations. Embracing diagnostic stewardship together with innovative technologies will ensure timely interventions and effective antimicrobial use.
References
1. Sexually transmitted disease surveillance, 2021. Cdc.gov. Published April 11, 2023. Accessed August 22, 2023. https://www.cdc.gov/std/statistics/2021/default.htm.
2. National overview of STDs, 2021. Cdc.gov. Published May 16, 2023. Accessed August 22, 2023. https://www.cdc.gov/std/statistics/2021/overview.htm.
3. Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187.doi:10.15585/mmwr.rr7004a1.
4. Mycoplasma genitalium. Cdc.gov. Published December 5, 2022. Accessed August 22, 2023. https://www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm.
5. Torrone EA, Kruszon-Moran D, Philips C, et al. Prevalence of Urogenital Mycoplasma genitalium Infection, United States, 2017 to 2018. Sex Transm Dis. 2021;1;48(11):e160-e162. doi:10.1097/OLQ.0000000000001394.
6. Herbst de Cortina S, Bristow CC, Joseph Davey D, Klausner JD. A Systematic Review of Point of Care Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Infect Dis Obstet Gynecol. 2016;2016:4386127. doi:10.1155/2016/4386127.
7. Caruso G, Giammanco A, Virruso R, Fasciana T. Current and Future Trends in the Laboratory Diagnosis of Sexually Transmitted Infections. Int J Environ Res Public Health. 2021;18(3):1038. doi:10.3390/ijerph18031038.
8. Gaydos CA, Van Der Pol B, Jett-Goheen M, et al. Performance of the Cepheid CT/NG Xpert Rapid PCR Test for Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2013;51(6):1666-1672. doi:10.1128/JCM.03461-12.
9. Adamson PC, Klausner JD. Diagnostic Tests for Detecting Chlamydia trachomatis and Neisseria gonorrhoeae in Rectal and Pharyngeal Specimens. J Clin Microbiol. 2022;60(4):e0021121. doi:10.1128/JCM.00211-2.
10. Gaydos CA, Manabe YC, Melendez JH. A Narrative Review of Where We Are With Point-of-Care Sexually Transmitted Infection Testing in the United States. Sex Transm Dis. 2021;48(8S):S71-S77. doi:10.1097/OLQ.0000000000001457.
11. Food and Drug Administration. Xpert CTNG Premarket Notification – 510(K) Substantial Equivalence Determination Decision Summary.
12. Shipitsyna E, Unemo M. A profile of the FDA-approved and CE/IVD-marked Aptima Mycoplasma genitalium assay (Hologic) and key priorities in the management of M. genitalium infections. Expert Rev Mol Diagn. 2020;20(11):1063-1074. doi:10.1080/14737159.2020.1842198.
13. Alinity m STI Assay. Abbot. Accessed August 13, 2023. https://www.molecular.abbott/int/en/products/infectious-disease/alinity-m-sti-assay.
14. CDC. The biggest antibiotic-resistant threats in the U.S. Centers for Disease Control and Prevention. Published July 15, 2022. Accessed August 22, 2023. https://www.cdc.gov/drugresistance/biggest-threats.html.
15. Gonococcal infections among adolescents and adults. Cdc.gov. Published December 5, 2022. Accessed August 22, 2023. https://www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm.
16. Klausner JD, Bristow CC, Soge OO, et al. Resistance-Guided Treatment of Gonorrhea: A Prospective Clinical Study. Clin Infect Dis. 2021;15;73(2):298-303. doi:10.1093/cid/ciaa596.
17. Allan-Blitz LT, Wang X, Klausner JD. Wild-Type Gyrase A Genotype of Neisseria gonorrhoeae Predicts In Vitro Susceptibility to Ciprofloxacin: A Systematic Review of the Literature and Meta-Analysis. Sex Transm Dis. 2017;44(5):261-265. doi:10.1097/OLQ.0000000000000591.
18. Allan-Blitz LT, Adamson PC, Klausner JD. Resistance-Guided Therapy for Neisseria gonorrhoeae. Clin Infect Dis. 2022;29;75(9):1655-1660. doi:10.1093/cid/ciac371.
19. Peterson SW, Martin I, Demczuk W, et al. Molecular Assay for Detection of Genetic Markers Associated with Decreased Susceptibility to Cephalosporins in Neisseria gonorrhoeae. J Clin Microbiol. 2015;53(7):2042-2048. doi:10.1128/JCM.00493-15.
20. Getman D, Jiang A, O'Donnell M, Cohen S. Mycoplasma genitalium Prevalence, Coinfection, and Macrolide Antibiotic Resistance Frequency in a Multicenter Clinical Study Cohort in the United States. J Clin Microbiol. 2016;54(9):2278-2283. doi:10.1128/JCM.01053-16.
21. Gaydos CA. Mycoplasma genitalium: Accurate Diagnosis Is Necessary for Adequate Treatment. J Infect Dis. 2017;216(suppl_2):S406-S411. doi:10.1093/infdis/jix104.
22. Jensen JS. Protocol for the detection of Mycoplasma genitalium by PCR from clinical specimens and subsequent detection of macrolide resistance-mediating mutations in region V of the 23S rRNA gene. Methods Mol Biol. 2012;903:129-139. doi:10.1007/978-1-61779-937-2_8.
23. Satyaputra F, Hendry S, Braddick M, Sivabalan P, Norton R. The Laboratory Diagnosis of Syphilis. J Clin Microbiol. 2021;59(10):e0010021. doi:10.1128/JCM.00100-21.
24. Syphilis Health Check. Product insert. Diagnostics Direct, LLC; 2015.
25. Bristow CC, Klausner JD, Tran A. Clinical Test Performance of a Rapid Point-of-Care Syphilis Treponemal Antibody Test: A Systematic Review and Meta-analysis. Clin Infect Dis. 2020;71(Suppl 1):S52-S57. doi:10.1093/cid/ciaa350.
26. DPP HIV-Syphilis System. Indications For Use. Chembio Diagnostic Systems Inc; 2020.
27. Shukla MR, Pereira L, Gaynor AM, et al. Evaluation of Three Automated Nontreponemal Rapid Plasma Reagin (RPR) Tests for the Laboratory Diagnosis of Syphilis. J Clin Microbiol. 2023;20;61(6):e0016823. doi:10.1128/jcm.00168-23.
28. FDA. 2018. 510(k) substantial equivalence determination decision summary K173376 ASI evolution. Accessed August 22, 2023. https://www.accessdata.fda.gov/cdrh_docs/reviews/K173376.pdf.
29. ASI Evolution - automated RPR syphilis test. Arlingtonscientific.com. Accessed August 22, 2023. https://www.arlingtonscientific.com/asi-evolution.
30. The purpose of this submission is to show that the Gold Standard Diagnostics AIX1000 Rapid Plasma Reagin (RPR) Automated Test System (which consists of the Gold Standard Diagnostics RPR reagents and the AIX1000 Analyzer) is substantially equivalent to the Arlington Scientific Inc. (ASI) RPR Card Test for syphilis on the ASiManager-AT Analyzer. Fda.gov. Accessed August 22, 2023. https://www.accessdata.fda.gov/cdrh_docs/reviews/K150358.pdf.
31. AIX 1000. Gsdx.us. Accessed August 22, 2023. https://www.gsdx.us/aix-1000.
32. FDA. 2017. 510(k) substantial equivalence determination decision summary K170413 BioPlex 2200 syphilis total & RPR Kit. Accessed August 22, 2023. https://www.accessdata.fda.gov/cdrh_docs/reviews/K170413.pdf.
33. BioPlex 2200 syphilis total & RPR. Bio-Rad Laboratories. Accessed August 22, 2023. https://www.bio-rad.com/en-us/product/bioplex-2200-syphilis-total-rpr?ID=ORHUVS4VY.
34. Vargas SK, Qquellon J, Vasquez F, et al. Laboratory Evaluation of the DPP Syphilis Screen & Confirm Assay. Microbiol Spectr. 2022;10(3):e0264221. doi:10.1128/spectrum.02642-21.
35. CDC awards Chembio $3.2M for development of syphilis POC test. Labpulse.com. Published September 6, 2022. Accessed August 22, 2023. https://www.labpulse.com/diseases/infectious/syphilis/article/15297580/cdc-awards-chembio-32m-for-development-of-syphilis-poc-test.
36. Doron S, Davidson LE. Antimicrobial stewardship. Mayo Clin Proc. 2011;86(11):1113-1123. doi:10.4065/mcp.2011.0358.
37. Programmes STI. The diagnostics landscape for sexually transmitted infections. Who.int. Published July 20, 2023. Accessed August 22, 2023. https://www.who.int/publications/i/item/9789240077126.
38. Claeys KC, Johnson MD. Leveraging diagnostic stewardship within antimicrobial stewardship programmes. Drugs Context. 2023;20;12:2022-9-5. doi:10.7573/dic.2022-9-5.