Best practices in preanalytical sample quality: Managing and preventing in vitro hemolysis in the emergency department
Laboratory errors can lead to errors in diagnosis and may cause increased costs, unnecessary testing and/or treatment, and adverse patient outcomes. Approximately 60–70% of laboratory errors are due to preanalytical factors.1 In vitro hemolysis is by far the most common preanalytical problem, accounting for 40–70% of unsuitable blood samples.2-4
Hemolysis is defined as erythrocyte rupture, where red blood cell contents are released into the bloodstream.5 It can occur in vitro or in vivo. In vitro hemolysis is substantially more common and can cause spurious changes in the plasma or serum concentration of potassium and other substances released from the damaged red blood cell.5
Causes of in vitro hemolysis
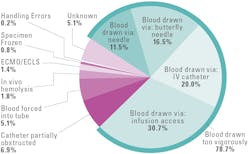
The majority of in vitro hemolysis results from inappropriate techniques for collecting, handling, or transporting blood specimens.4,6 Table 1 details preanalytical errors that can cause hemolysis, with incorrect sample collection being the most common. Figure 1 details causes and frequencies of hemolysis in statistical/urgent testing when the healthcare provider requires test results as quickly as possible.
Prevalence of hemolysis
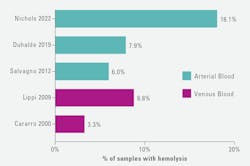
Hemolysis occurs frequently in samples obtained in the emergency department (ED), with reported rates ranging up to 18.1% in arterial blood gas samples, and in 8.8% of venous blood samples (Figure 2).8-12
Central laboratory chemistry analyzers typically include automated spectrophotometric hemolysis detection and report a semiquantitative hemolysis index (HI).13,14 Hemolysis detection capabilities are much less common in point-of-care testing (POCT) analyzers commonly used in the ED. As a result, hemolysis in the ED can go undetected.14,15
Impact of hemolysis
Release of potassium and other substances from hemolyzed red blood cells can affect laboratory tests and cause reporting of erroneous results by the following:16
· Elevating plasma levels of potassium and other substances contained within the red blood cells
· Diluting the plasma
· Causing spectral interference
· Releasing enzymes that degrade analytes of interest
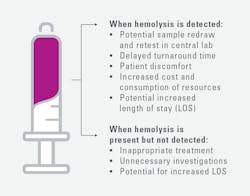
Hemolysis can require sample recollection, delay care, and increase costs. If hemolysis is not detected, inappropriate treatment and unnecessary investigations may result (Figure 3).16
Potassium is a key analyte for POCT in the ED because potassium abnormalities are common in patients in this setting and can cause life-threatening cardiac arrhythmias and other complications. Hemolysis can cause spurious elevations in plasma potassium levels. Because potassium levels are typically at least 20-fold higher in red blood cells than in plasma, even minor hemolysis can result in pseudohyperkalemia or mask hypokalemia.17 Several studies illustrate the potential clinical impact.
· A study evaluated potassium levels and hemolysis index (HI) in 550 arterial blood gas samples. Of these, whole blood potassium levels were considered hypokalemic (<3.5 mmol/L) in 150 samples, normokalemic (3.5-4.5 mmol/L) in 328 samples, and hyperkalemic (>4.5 mmol/L) in 72 samples. 18% of samples had an HI >1, representing at least mild hemolysis. Correcting for the effects of hemolysis led to downgrading 22% of normokalemic samples and 14% of hyperkalemic samples. Hemolysis was associated with low sample volume (<0.5 mL).18
· In an ED-based study of POCT in 100 unique ED admissions with elevated potassium, comparison of POC and central lab results found that 40 of the elevated potassium measurements represented pseudohyperkalemia due to hemolysis (39 patients) or contamination with IV fluid (1 patient). Review of medical records indicated that pseudohyperkalemia affected care in 11 patients, including 6 with unnecessary follow-up testing, 3 with delayed treatment, and 2 with inappropriate interventions.19
· Another ED-based study analyzed 472 arterial blood gas samples at POC for hemolysis and potassium. Of these, 12% were hemolyzed. Potassium levels were significantly higher in hemolyzed than in non-hemolyzed samples, with an increase of 15.29% (0.61 mEq/L), P<0.001.20 The authors recommended making POCT hemolysis detection an integral part of the diagnostic process.20
· A study of 3,185 ED samples evaluated for hemolysis by visual inspection found that 495 samples (15.5%) were hemolyzed. Of the 2,518 tests ordered for these hemolyzed samples, 780 results (31%) were handled incorrectly (either incorrectly released or incorrectly suppressed).12 The tests with the highest risk for patient safety (i.e., those with high rates of hemolysis and high risk of patient harm) were potassium, troponin T, and total bilirubin.12
These errors can have considerable clinical and economic impact. A series of studies by Phelan et. al. estimated that hemolysis in the ED was associated with a 62-minute increase in a patient’s LOS in the ED.21 For a hypothetical typical ED, the direct cost of this increased LOS is estimated at $4 million per year. The ED was assumed to have 100,000 annual visits, with 40% of patients undergoing a blood draw for routine chemistry testing, and a 10% incidence of hemolysis for each blood draw.22
True hyperkalemia is rare among low-risk patients. A study of ED encounters, with 66 low-risk patients who presented with elevated potassium levels and hemolysis on initial testing, found that no patients had true hyperkalemia in a repeat test.23 Low-risk patients were defined as 18–65 years of age, not taking potassium-altering medications, had no hematologic malignancies, and had a repeat test within 12 hours.23 The median time between the initial and repeat blood draws was 145 minutes, representing an opportunity to decrease the ED LOS.23
Detection and reporting
Historically, EDs have been unable to detect hemolysis routinely at the POC. For whole blood potassium measurement, they may rely instead on clinical judgement, sample recollection, and/or HI measurement in the central lab. The lack of hemolysis detection presents several clinical challenges. These can be mitigated by POCT hemolysis testing (Figure 3). In recent years, stand-alone POCT hemolysis analyzers and a POCT blood gas analyzer with an integrated hemolysis detection capability have become available.
The prevalence and impact of hemolysis and the limitations of earlier approaches to hemolysis detection have led to calls for routine inclusion of hemolysis detection functionality in blood gas analyzers.24 In addition, the Clinical & Laboratory Standards Institute (CLSI) EP23 guideline notes that risk evaluation should include the ability to detect erroneous results and prevent medical action before they cause patient harm; and, that some hazards can be detected internally by the measurement system.25 The measurement system’s ability to detect hemolysis is an important consideration.25
Conclusion
Hemolysis is a common problem in ED POCT analyses and can lead to spurious elevations in plasma levels of potassium and other analytes, potentially requiring sample recollection, delays, increased costs, and unnecessary procedures and treatment. Incorporation of automated hemolysis detection into routine POCT analyses can help mitigate these risks and improve patient safety (Figure 4).
References
- Lippi G, Chance JJ, Church S, et al. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 2011;49(7):1113-26. doi:10.1515/CCLM.2011.600.
- Plebani M. The detection and prevention of errors in laboratory medicine. Ann Clin Biochem. 2010;47(Pt 2):101-10. doi:10.1258/acb.2009.009222.
- Lippi G, Salvagno GL, Favaloro EJ, Guidi GC. Survey on the prevalence of hemolytic specimens in an academic hospital according to collection facility: opportunities for quality improvement. Clin Chem Lab Med. 2009;47(5):616-8. doi:10.1515/CCLM.2009.132.
- Lippi G, Avanzini P, Pavesi F, et al. Studies on in vitro hemolysis and utility of corrective formulas for reporting results on hemolyzed specimens. Biochem Med (Zagreb). 2011;21(3):297-305. doi:10.11613/bm.2011.040.
- Buño A, Oliver P. POCT errors can lead to false potassium results. Adv Lab Med. 2021;30;3(2):142-152. doi:10.1515/almed-2021-0079.
- Lippi G, Blanckaert N, Bonini P, et al. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin Chem Lab Med. 2008;46(6):764-72. doi:10.1515/CCLM.2008.170.
- Carraro P, Servidio G, Plebani M. Hemolyzed specimens: a reason for rejection or a clinical challenge? Clin Chem. 2000;46(2):306-7.
- Nichols JH, Apple FS. Prevalence of Hemolyzed Whole Blood Potassium Results in Acute Care Settings. 28th AACC International CPOCT Symposium.; 2022.
- Duhalde H, Skogö J, Karlsson M. Point-of-care hemolysis detection in blood gas specimens directly at the emergency department. Scand J Clin Lab Invest. 2019;79(5):283-287. doi:10.1080/00365513.2019.1612089.
- Salvagno GL, Lippi G, Gelati M, Guidi GC. Hemolysis, lipaemia and icterus in specimens for arterial blood gas analysis. Clin Biochem. 2012;45(4-5):372-3. doi:10.1016/j.clinbiochem.2011.12.005.
- Lippi G, Plebani M, Di Somma S, Cervellin G. Hemolyzed specimens: a major challenge for emergency departments and clinical laboratories. Crit Rev Clin Lab Sci. 2011;48(3):143-53. doi:10.3109/10408363.2011.600228.
- Luksic AH, Nikolac Gabaj N, Miler M, et al. Visual assessment of hemolysis affects patient safety. Clin Chem Lab Med. 2018;28;56(4):574-581. doi:10.1515/cclm-2017-0532.
- Farrell CJ, Carter AC. Serum indices: managing assay interference. Ann Clin Biochem. 2016;53(Pt 5):527-38. doi:10.1177/0004563216643557.
- Dolci A, Panteghini M. Harmonization of automated hemolysis index assessment and use: Is it possible? Clin Chim Acta. 2014;15;432:38-43. doi:10.1016/j.cca.2013.10.012.
- Simundic AM, Topic E, Nikolac N, Lippi G. Hemolysis detection and management of hemolyzed specimens. Biochem Med. 2010;20(2):154-159.
- McCaughey EJ, Vecellio E, Lake R, et al. Current Methods of Haemolysis Detection and Reporting as a Source of Risk to Patient Safety: a Narrative Review. Clin Biochem Rev. 2016;37(4):143-151.
- Beilin LJ, Knight GJ, Munro-Faure AD, Anderson J. The sodium, potassium, and water contents of red blood cells of healthy human adults. J Clin Invest. 1966;45(11):1817-25. doi:10.1172/JCI105485.
- Hawkins R. Measurement of whole-blood potassium--is it clinically safe? Clin Chem. 2003;49(12):2105-6. doi:10.1373/clinchem.2003.027227.
- O'Hara M, Wheatley EG, Kazmierczak SC. The Impact of Undetected In Vitro Hemolysis or Sample Contamination on Patient Care and Outcomes in Point-of-Care Testing: A Retrospective Study. J Appl Lab Med. 2020;1;5(2):332-341. doi:10.1093/jalm/jfz020.
- Nigro M, Valli G, Marchionne ML, et al. Is There a Risk of Misinterpretation of Potassium Concentration from Undetectable Hemolysis Using a POCT Blood Gas Analyzer in the Emergency Department? Medicina (Kaunas). 2022;28;59(1):66. doi:10.3390/medicina59010066.
- Phelan MP, Hustey FM, Good DM, Reineks EZ. Seeing Red: Blood Sample Hemolysis Is Associated with Prolonged Emergency Department Throughput. J Appl Lab Med. 2020;1;5(4):732-737. doi:10.1093/jalm/jfaa073.
- Phelan MP, Ramos C, Walker LE, Richland G, Reineks EZ. The Hidden Cost of Hemolyzed Blood Samples in the Emergency Department. J Appl Lab Med. 2021;1;6(6):1607-1610. doi:10.1093/jalm/jfab035.
- Wilson M, Adelman S, Maitre JB, et al. Accuracy of Hemolyzed Potassium Levels in the Emergency Department. West J Emerg Med. 2020;20;21(6):272-275. doi:10.5811/westjem.2020.8.46812.
- Möckel M, Luppa PB. Why hemolysis detection should be an integral part of any near-patient blood gas analysis. Journal of Laboratory Medicine. 2021;45(4-5):193-195. doi:10.1515/labmed-2021-0076.
- CLSI. Laboratory Quality Control Based on Risk Management. 2nd ed. CLIS guideline EP23™. Clinical and Laboratory Standards Institute; 2023.