Electrolytes in blood (and water): Measurement and clinical overview
To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe what electrolytes are and the elements that constitute them.
2. Differentiate which electrolytes make up the different homeostatic systems.
3. Describe the advancements of measurement technology in enhanced blood gas analyzers and their limitations.
4. Discuss clinical implications of electrolyte imbalances using the anion gap.
Electrolytes are simply electrically charged molecules or elements (ions) that get their charge as they dissolve by complete or partial dissociation in an aqueous medium — that is, in the water fraction of the fluid in which they are a part. Acids, bases, proteins, salts, and solids can be electrolytes under a specific set of circumstances. For example, table salt or sodium chloride (NaCl), dissolves by dissociation into sodium ion and chloride ion with no remaining sodium chloride molecule in the solution: NaCl à Na+ + Cl-. However, carbonic acid (dihydrogen carbonate) partially dissociates into hydrogen ions and hydrogen carbonate ions (bicarbonate ions) with small amounts of the acid molecule remaining: H2CO3 ⇌ H+ + HCO3-.
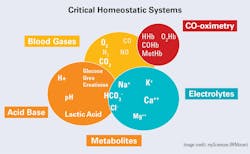
Our focus here is the electrolytes of blood, primarily those that are major determinants of water and electrical balance within the homeostatic system (Figure 1). Other essential electrolytes (e.g., calcium, magnesium, bicarbonate, phosphate) are best covered in detail separately.
Electrolytes play several major roles in sustaining life. The complexity of the human organism is such that all these aspects can play a role in maintaining overall electrical neutrality/electrochemical signal transmission and osmotic (cellular) balance. Further, some are essential to enzyme action and energy production when they function as co-factors in critical reactions. For the sake of brevity and with a focus on urgent care (e.g., point-of-care or POC), this discussion of electrolytes will take a simple approach.
Among the common electrolytes, sodium (Na) and potassium (K), are the principal cations (positive ions) in the extracellular and intracellular fluids, respectively. Jointly, they help to keep both electrical neutrality and cellular integrity, along with their counterpart anions (negative ions), chloride (Cl), and bicarbonate (HCO3-). Calcium(Ca) and magnesium (Mg) are significant in bone structure and a small fraction of each, as ions, are critical to functions such as energy production and cardiac function. Finally, proteins/proteinate (PR-) anions are contributors to ionic balance as well as to cell integrity because of their osmotic pressure.
Measurement technology affects clinical decisions
Clinical evaluation in the acute care setting requires both testing of body fluids for electrolyte levels and clinical diagnostic/therapeutic intervention activities. Therefore, the POC measurement’s capabilities must be not only rapid but as exact as central laboratory testing. The enhanced blood gas analyzers (eBGAs) as currently marketed meet that criterion, but in doing so, raise some issues that may not be fully appreciated by caregivers/physicians. These issues can impact terminology as well as clinical practice and judgment. Measurement of electrolytes in whole blood is an extension of the blood gas technology itself. That is, membranes cover the sensor to prevent cells from interfering and each electrolyte is sensed because of its quantitative interaction with material in or behind the membrane which in turn causes a change in voltage or current. While specific components may differ, the key is that the sensor detects the signal that results from the concentration in the water fraction. On the other hand, most central laboratory systems dilute the specimen and aqueous calibrator before measurement, meaning that the presence of plasma/serum protein, which is not part of the fluid in which the electrolyte is dissolved, is ignored!
From the earliest days of measurement, amounts of electrolyte reported were based on the volume of liquid (serum or plasma), not on the liquid in which the electrolyte is dissolved. The result is thus factitiously lower. Fortunately, this does not matter if all patients have the same protein levels (6–8% of serum/plasma volume). But as is well known, protein concentrations vary and protein abnormalities exist, and they can affect the apparent electrolyte results. There is a comparable situation that can exist in situations of high lipid levels — extremely low results can be reported using the older technology.
Modern technology wins. The electrolyte sensors in the eBGAs are selective for the concentration/activity in the water part of the whole blood. One added note to this issue, the eBGAs of at least the three major manufacturers (Siemens, Radiometer, and Werfen) are all designed to report the electrolytes with reference values based on normal plasma water (i.e., they are ‘harmonized’ to agree using the protocol of CLSI standard C29A2). So, despite the direct measurement technology, no meaningful change in reference values for electrolytes would be manifested. This was developed out of concern for potential confusion and issues with clinical care through a consensus process with the Clinical and Laboratory Standards Institute (CLSI), the National Institute of Standards and Technology (NIST), and the scientists of several manufacturers of systems having this technology.
Clinical implications
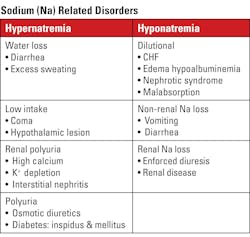
The two major ions of extracellular fluid (EC), especially of blood plasma, are sodium and chloride. These ions track each other in health and abnormal conditions with a few notable exceptions, such as metabolic acidosis, some cancer medications, and fluid replacement treatment (See Table 2).
Potassium is a cation like sodium but in a much lower concentration, and it is not a direct/major player in fluid balance. (Its role is due more to its effects on heart muscle.)
Sodium
Sodium’s typical values are 135–148 mmol/L, a narrow range. Deviations outside of < 120 mmol/L or > 155 may be life-threatening, but even small deviations outside the reference range may cause significant clinical changes. Note on unit used: The recommended unit of concentration is mol/L (molarity) or one of its fractions, usually millimole per liter (mmol/L). However, some reports use mEq/L. The numeric value for all monovalent ions is the same using either unit.
Specimen collection: Routine electrolytes are typically measured on serum(s). However, with the proper anticoagulant, plasma may be used. In critical care, whole blood collected in syringes, evacuated tubes, or capillary tubes (anatomic sources: arterial, venous, capillary bed) are used but the ion-selective electrodes (ISE) functionally separate the cells from liquid and the values are concentrations in plasma (p) or plasma-water (ph) harmonized to agree with the central lab method. Using whole blood reduces processing and waiting time, and if blood gases are required, eliminates a separate collection. However, collection devices must have standardized volumes of liquid heparin (usually lithium heparin to avoid other interferences) or very soluble dry/lyophilized (‘crystallized’) heparin. Caveat: The use of therapeutic heparin is likely to cause errors, due to specimen dilution variation and/or electrolyte/pH effects from the heparin used (e.g., Na-heparin).
Clinical implications: Sodium is the major extracellular cation. As such, it controls the fluid space and balance and consequently osmotic relationships. See Table 1 for conditions related to too high of concentrations (hypernatremia) and too low of concentrations (hyponatremia) in the blood.
Chloride
The element/ion chloride is the major extracellular anion. (atomic number of 17, atomic mass 35.5 g/mol.). Chloride levels parallel those of the sodium ion (Na+). Typical values for chloride are between 95–110 mmol/L. Again, as with sodium, the concentration may be reported as mEq/L, with no numeric difference.
Specimen collection: Identical to sodium.
Chloride measurement: Early measurement technology relied on the reaction of the chloride ion present in the specimen with either silver to form a precipitate of AgCl, or with a chelating agent to form a colored complex with later titration to obtain results. Most eBGA’s are based on ISEs with the sensor having a chloride-sensitive chromophore. The key is the selectivity of the sensor for chloride; that is the chromophore reaction with chloride only.
Clinical implications: An elevated or decreased chloride measurement can indicate several primary conditions as well as those that are secondary to other disorders (See Table 2).
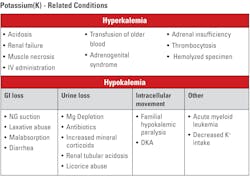
Potassium
Potassium (K+) in the blood plasma/serum is a minor cation with a major impact. With typical values between 3.5–4.6 mmol/L, it is between two and three percent of the cationic load. However, as seen in Table 3, minor numerical changes have grave consequences. Potassium has the same unit (mmol/L) and the same specimen handling requirements as sodium and chloride, and using eBGA’s, it is also measured in plasma water. The fact that potassium is found in high concentrations in intact cells leads to the need for exceptional care in collecting specimens to avoid tissue or blood cell potassium introduction to the specimen by excessive manipulation of the limb during collection or of the specimen itself, leading to hemolysis, both of which may be factors in the values obtained (i.e., artefactually elevated).
Potassium measurement: Technology for potassium measurement has evolved along with other electrolytes. Originally, potassium was measured by techniques like that of sodium. Today, the eBGA’s measurements are based on ISEs with the sensor having a potassium-sensitive chromophore rather than the glass electrode used for sodium; other than that, the procedure is the same.
Clinical implications: Differing potassium values are shown in Table 3. Both elevated (hyperkalemia) and decreased (hypokalemia) potassium levels can affect important functions in the body.
Anion Gap
The anion gap (AnGap or A.G.) blood test is the difference between the ‘total’ concentration of cations and the measured number of anions and has been used as a diagnostic tool to differentiate between causes of electrolyte and acid-base disorders. On most eBGAs, the cations used are sodium and potassium and the anions are the measured chloride and the bicarbonate (hydrogen carbonate) from the blood gas measurements since all are readily available on the same measurement platform (See Figure 2).
Clinical Implications: The anion gap calculation aids in the assessment of non-respiratory/metabolic acidosis. A high anion gap usually shows the presence of some combination of the unmeasured anions already mentioned, as well as sulfate, acetoacetate, β-hydroxybutyrate, etc. Sources of these anions must be part of the evaluation of acidosis such as diabetic ketoacidosis (DKA) or alcohol abuse. Toxins such as methanol, glycol, iron, cyanide, aspirin, etc. or uremia/renal failure with decreased bicarbonate reabsorption, decreased acid excretion, accumulation of sulfates, urates, and phosphates should also be considered.
Normal AnGap with acidosis can only be the result of decreased bicarbonate. To keep neutrality, chloride must increase as it is the only major anion. Loss of bicarbonate may be caused by GI loss (diarrhea), Renal loss (renal tubular acidosis), ingestions (ammonium chloride), or total parenteral nutrition (TPN).
Low AnGap can result from loss of albumin or from an increase in chloride and bicarbonate to keep neutrality.
Electrolytes: An overall summary
The full scope of a discussion on electrolytes, both the analytical and the clinical, deserves much more space than we have here. But even in the brief paragraphs above, it is evident that electrolytes have a dramatic clinical impact on the overall well-being of the patient. The clinician must consider not only whether there is a primary electrolyte disturbance or one related to other therapeutic interventions. Especially when there is a discrepancy between the eBGA electrolytes and the central laboratory’s results, such differences must be clinically evaluated in addition to investigation of potential analytical error. The electrolyte homeostatic systems are related to the acid-base, and all are related to the pulmonary gas exchange, oxygen transport, and utilization.
References/Suggested Readings
- Clinical and Laboratory Standards Institute. Approved Standard C29-A2. Clinical and Laboratory Standards Institute; 2000. Wayne, PA. Available from: https://clsi.org/media/1364/c29a2_sample.pdf.
- Moran RF, Liesching TN. The ABC’s of Abg’s(Tm): A Cyclopedic Dictionary of the Testing Terms Used in Critical Care. Momentum Press; 2018.
- Moran RF. POC Testing and Reporting of Sodium, and Other Small Molecules Need Modified IFCC Source/Type Designations to Improve Operational Efficacy and for Clinically Accurate, Unambiguous Reporting from LIMS and HIS. EJIFCC. 2023;34(4):271-275.
- Moran RF. CE: Point of Care-Managing change when you are not in charge. MLO. 2024;56:8-12.
To take the test online go HERE. For more information, visit the Continuing Education tab.
About the Author

Robert F. Moran, PhD, FCCM, FIUPAC
is the Principal Scientist at mviSciences, a consulting and educational services organization and President of AccuTest™ Proficiency Testing Services. Dr. Moran served multiple terms on the NCCLS (Now CLSI) Board of Directors and was an active participant or chairholder in several of their blood gas and electrolyte standards-writing teams. Also active in clinical chemistry internationally, he is an appointed Fellow of the International Union of Pure and Applied Chemistry (FIUPAC). He is a retired professor of chemistry and physics from Wentworth Institute of Technology but remains active in consulting work and writing.