In a world overpopulated by pathogens, the human immune system has evolved to protect against pathogens that are, themselves, continually evolving. Host immune response to infection refers to the dynamic process that maintains the delicate balance between effective pathogen clearance and minimizing damage to the host tissues that allows the body to recognize and fight infections. However, when the system becomes dysregulated, overreacting to invaders, it can lead to sepsis, septic shock, and ultimately death.
With nearly 50 million yearly global cases of sepsis and 11 million sepsis-related deaths,1 the need for diagnostic tests that quickly identify infection and immune dysregulation is obvious. This clinical need has inspired significant innovations over the past decade, which are now emerging as regulatory-cleared diagnostic products and solutions.
Innate immunity diagnostics: Targeting the first line of defense
The host immune response is a diverse, complex, and dynamic defense system that plays a crucial role in protecting the body against infectious agents. When an infection occurs, the immune system is activated to identify and eliminate the pathogen. This response involves coordinated efforts between innate and adaptive immune components, as well as various immune cells, molecules, and signaling pathways, working together for an effective defense.
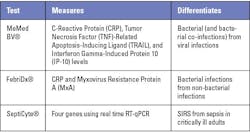
Each of these components, to varying degrees, could serve as diagnostic targets for the presence of infection and/or indicate infection severity. Based on these diagnostic targets, multiple tests have been developed, such as those in Table 1.
One of the challenges in implementing these tests is that they require an additional order when there is diagnostic uncertainty or suspicion of sepsis. However, many patients present to the emergency department (ED) with vague signs and symptoms that do not align with sepsis screening criteria. In a study of over 2,100 ED patients that ultimately developed sepsis within 12 hours of presentation, 33% did not meet SIRS criteria and 88% did not meet qSOFA criteria.2 Accordingly, for these tests to aid in sepsis management, they would need to be performed routinely during triage and provide useful insights to support clinical decisions. For example, MeMed BV rapidly and accurately discerns bacterial from viral infections in suspected sepsis patients,3 potentially leading to earlier initiation of appropriate care in the ED. One of the challenges in implementing these tests, however, is that they require an additional order when there is diagnostic uncertainty or suspicion of sepsis.
One of the first and most commonly ordered tests in the ED is the complete blood count with differential (CBC-diff).4–6 Significant advances in hematology analyzers have expanded its capabilities so that the tried-and-true technique can be applied to enhance severe infection screening.
White blood cell activation
The automated differential is a cornerstone of modern medicine. In the context of host immune response, an abnormal white blood cell count is among the first signals of infection and immune dysregulation in many clinical scenarios. Absolute count and percentages of the five normal blood cell types provide a wealth of clinical insights. High band counts (immature neutrophils) and elevated immature granulocyte levels can signal severe infection. The neutrophil to lymphocyte ratio (NLR) is recognized as a marker for infection and inflammatory stress indicative of severe illness. Absolute count and percentages of metamyelocytes, myelocytes, and promyelocytes suggest immune response to bacterial illness.
More recently, some hematology analyzers have introduced enhanced volume measurements, permitting automated assessment of morphological characteristics. For example, monocyte distribution width (MDW) is a regulatory-cleared hematological parameter that measures variation in monocyte size.7 MDW values of ≥20 (K2EDTA) or ≥21.5 (K3EDTA) are associated with a higher risk of developing sepsis within the first 12 hours of hospital admission. MDW elevation is pathogen agnostic,8 meaning that it will respond similarly to bacterial, viral, and fungal agents. Interpreting MDW in the context of other leukocyte parameters can enhance diagnostic accuracy. For example, many studies have demonstrated strong diagnostic performance of elevated MDW and abnormal WBC as compared with any single leukocyte parameter.9–11 In one study, patients with elevated MDW, abnormal WBC count, and elevated NLR were 12 times more likely to develop sepsis and 9.3 times more likely to develop septic shock.12
Sepsis, like many serious conditions, often presents with vague symptoms, but without prompt treatment, septic patients can rapidly deteriorate. Thus, identifying and validating novel biomarkers to assist in accurate disease diagnosis is important to help in early sepsis detection and timely treatment. Using MDW, abnormal WBC count, and NLR in combination has shown superior diagnostic performance over any single parameter, including among patients who presented to the ED without overt sepsis symptoms.13,14
Maximize clinical insight, minimize microscopy
Like monocytes, each white blood cell type undergoes specific morphological and functional changes during the host immune response. Neutrophils increase in number and, in some severe infections, can present cytoplasmic toxic granules and vacuoles, eosinophils show increased cytoplasmic vacuolization, and monocytes, in addition to changing in size, show large coalescing cytoplasmic vacuoles that can be visualized under light microscopy.15,16 However, conducting a manual review to identify these features is too time consuming for routine clinical screening or as part of emergent care.15,16 The advent of digital hematology systems have provided the power to review, detect, and identify cells in full context with AI-powered image recognition and clinical decision support technology without reverting to the microscope.17,18
Automated hematology analyzers will never fully replace manual slide review. Manual review is still required to fully characterize cell abnormalities and establish whether these abnormalities are likely associated with infection or some other cause. However, recent advances in digital hematology will minimize microscope time and enable streamlined slide review. And hematology automation innovations introduced by companies such as Scopio® provide integrated hardware and software solutions to streamline the transition from automatic differential to manual review.
The next generation of digital pathology imaging incorporates larger fields of view in high resolution, as well as AI decision support and off-site reviews. For example, the Scopio Lab Full-Field Peripheral Blood Smear® application provides a view of all clinically relevant areas of the blood smear, including the monolayer and feathered edge, at 100X magnification. This configuration supported remote viewing for a tertiary medical center hematology division that reduced turnaround time while maintaining overall workload and clinical quality. Retrospective analysis of 10,704 peripheral blood smear samples were analyzed pre- and post-implementation over a 5-month period. Incorporating effective remote viewing, where pathologists were able to conduct satisfactory peripheral blood smear slide review from home, resulted in an overall 15.8% reduction in turnaround time for the entire lab.
These capabilities could provide a new opportunity to evaluate additional aspects of white blood cells to determine the presence and severity of infection. As AI cell typing matures, the opportunities for assistance in cell classification, disease identification, and response to therapies will grow.19,20
Conclusion
Continued study of the intricacies of the host response to infection is facilitating novel diagnostic strategies to identify disease presence and severity using results from the CBC-diff and other blood biomarkers. These advances are changing diagnostics, providing opportunities to improve routine clinical care and give clinicians valuable tools to quickly identify infections and immune dysregulation, leading to improved patient outcomes. Diagnostic tests that measure host immune response biomarkers have shown promise for earlier sepsis detection as well as in differentiating between bacterial and viral infections. Additionally, advances in hematology analyzers, including enhanced volume measurements and AI-powered image recognition, allow for more accurate and efficient blood analysis. The integration of AI support in digital hematology imaging with CBC-diff results and other patient information holds great potential for further advancements in cell classification and disease identification—all with less than 0.5 mL of blood.
By leveraging these evolving complementary technologies, discovering how they can be combined and protocolized and adopting future innovations as they become available, healthcare professionals can effectively triage, detect, and intervene, to deliver a higher standard of care in the battle against infections, from common colds to septic shock.
References
1. Sepsis. Who.int. Accessed March 4, 2024. https://www.who.int/health-topics/sepsis.
2. Crouser ED, Parrillo JE, Martin GS, et al. Monocyte distribution width enhances early sepsis detection in the emergency department beyond SIRS and qSOFA. J Intensive Care. 2020;5;8:33. doi:10.1186/s40560-020-00446-3
3. Angel A, Eden E, Avioz NZ, Gottlieb T, Navon R. 161 A host response test (MMBV) for differentiating between bacterial and viral infection has potential to improve antibiotic stewardship in patients with suspected sepsis. Ann Emerg Med. 2023;82(4):S72-S73. doi:10.1016/j.annemergmed.2023.08.183.
4. Young GP. CBC or not CBC? That is the question. Ann Emerg Med. 1986;15(3):367-371. doi:10.1016/s0196-0644(86)80587-x.
5. Rui P, Kang K. National Hospital Ambulatory Medical Care Survey: 2017 Emergency Department Summary Tables. U S Census Bureau. 2017;2017(37).
6. Barnes PW, McFadden SL, Machin SJ, Simson E, international consensus group for hematology. The international consensus group for hematology review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol. 2005;11(2):83-90. doi:10.1532/LH96.05019.
7. Crouser ED, Parrillo JE, Seymour C, et al. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest. 2017;152(3):518-526. doi:10.1016/j.chest.2017.05.039.
8. Piva E, Zuin J, Pelloso M, et al. Monocyte distribution width (MDW) parameter as a sepsis indicator in intensive care units. Clin Chem Lab Med. 2021;59(7):1307-1314. doi:10.1515/cclm-2021-0192.
9. Crouser ED, Parrillo JE, Seymour CW, et al. Monocyte distribution width: A novel indicator of Sepsis-2 and Sepsis-3 in high-risk emergency department patients. Crit Care Med. 2019;47(8):1018-1025. doi:10.1097/CCM.0000000000003799.
10. Hausfater P, Robert Boter N, Morales Indiano C, et al. Monocyte distribution width (MDW) performance as an early sepsis indicator in the emergency department: comparison with CRP and procalcitonin in a multicenter international European prospective study. Crit Care. 2021;25(1):227. doi:10.1186/s13054-021-03622-5.
11. Poz D, Crobu D, Sukhacheva E, et al. Monocyte distribution width (MDW): a useful biomarker to improve sepsis management in Emergency Department. Clin Chem Lab Med. 2022;60(3):433-440. doi:10.1515/cclm-2021-0875.
12. Malinovska A, Hinson JS, Badaki-Makun O, et al. Monocyte distribution width as part of a broad pragmatic sepsis screen in the emergency department. J Am Coll of Emerg Physicians Open. 2022;3(2):e12679. doi:10.1002/emp2.12679.
13. Hinson JS, Sarani N, Smith A, et al. A-155 Unlocking the complete blood count: derivation of a single-panel laboratory test that includes monocyte distribution width to create a universal sepsis screening tool. Clin Chem. 2023;69(Supplement_1). doi:10.1093/clinchem/hvad097.140.
14. Hinson J, Sarani M, Smith A, Badaki-Makun L, Malinovska A, Levin S. Unlocking the CBC: Derivation of a Single-Panel Laboratory Test That Includes Monocyte Distribution Width to Create a Universal Sepsis Screening Tool. In: Wiley; 2023. Accessed December 13, 2023. https://onlinelibrary.wiley.com/toc/15532712/2023/30/S1.
15. Zonneveld R, Molema G, Plötz FB. Analyzing neutrophil morphology, mechanics, and motility in sepsis: options and challenges for novel bedside technologies. Crit Care Med. 2016;44(1):218-228. doi:10.1097/CCM.0000000000001266.
16. Pozdnyakova O, Connell NT, Battinelli EM, et al. Clinical significance of CBC and WBC morphology in the diagnosis and clinical course of COVID-19 infection. Am J Clin Pathol. 2021;155(3):364-375. doi:10.1093/ajcp/aqaa231.
17. Katz B-Z, Benisty D, Sayegh Y, Lamm I, Avivi I. Remote digital microscopy improves hematology laboratory workflow by reducing peripheral blood smear analysis turnaround time. Appl Clin Inform. 2022;13(5):1108-1115. doi:10.1055/a-1957-6219.
18. Katz B-Z, Feldman MD, Tessema M, et al. Evaluation of Scopio Labs X100 Full Field PBS: The first high-resolution full field viewing of peripheral blood specimens combined with artificial intelligence-based morphological analysis. Int J Lab Hematol. 2021;43(6):1408-1416. doi:10.1111/ijlh.13681.
19. Walter W, Pohlkamp C, Meggendorfer M, et al. Artificial intelligence in hematological diagnostics: Game changer or gadget? Blood Rev. 2023;58:101019. doi:10.1016/j.blre.2022.101019.
20. Walter W, Haferlach C, Nadarajah N, et al. How artificial intelligence might disrupt diagnostics in hematology in the near future. Oncogene. 2021;40(25):4271-4280. doi:10.1038/s41388-021-01861-y.