As the population of diabetic and pre-diabetic patients continues to increase, the need for diabetic biomarkers for earlier diagnosis, more effective treatment monitoring and earlier indications of pending disease complications is becoming more critical. The need to standardize methods to ensure accurate results across clinical platforms will remain critical to patient diagnosis, classification and care.
Insulin resistance and diagnostic biomarkers
Looking to the future of diabetes management, consideration for the multitude of factors affecting insulin production, insulin resistance and ꞵ-cells will lead to a better understanding of how individualized treatment and monitoring may work. Research into the pathophysiology of diabetes has led to the suggestion of novel biomarkers for diagnosing and monitoring diabetes.3,4,5,6,7,8 The field of diabetic research has led to 10 Nobel prizes since 1923, and while diagnosis still relies heavily on glucose testing and HbA1c, the biological pathways involved in diabetes are complex and there may be a need for combination treatments for the best outcomes.1,2 Typically, by the time a patient is diagnosed with diabetes, they have lost some of their b-cell function and are exhibiting clinical indicators, providing more evidence that the need for earlier biomarkers is key to treating at risk patients.
Complications caused by diabetes are most concerning, as its effects on the heart, liver, kidneys, brain and eyes lead to irreversible damage and, in some cases, death. The disease’s pathophysiology has also lead to the discovery of a multitude of drugs for treating diabetes in conjunction with insulin or as a standalone treatment with life style changes. Metformin, an insulin sensitizing agent, is one such drug.
The exact mechanism through which insulin resistance occurs has not been identified; however, it has been identified that both overproduction of glucose and lack of glucose uptake play an integral role.1,2,7 While this review focuses primarily on novel clinical chemistry biomarkers for diagnosing and monitoring T2DM, it is notable that in the last decade, there have been multiple T2DM associated genes identified. In his review, Defronzo discusses transcription factor 7 like 2 (TCF7L2), which plays a role in b-cell production and insulin secretion. The t-allele of a single nucleotide polymorphism of the TCF7L2 gene is associated with impaired insulin secretion and both CT and TT genotypes can predict T2DM.2
All types of diabetes are complex and involve multiple tissues and organs, with subsequent downstream effects on organ systems and metabolic pathways.3 Around 2009, there was a switch from the typical Triumvirate theory to an Ominous Octet theory. The Ominous theory suggests that it is not only the muscles, liver and b-cells (triumvirate) that contribute to diabetic pathophysiology, but also adipocytes, pancreatic ꞵ-cells, and cells of the gastrointestinal tract, the kidney and the brain. Insulin sensitivity and uptake of glucose play critical roles in diabetes and yet it is still the failure of the b-cell that causes diabetes development to advance. The gold standard for measuring functioning b-cells is defined by: (change in insulin/change in glucose)/insulin resistance or [(ΔI/ΔG) ÷ IR].1,2,5 This switch to a multisystem theory, opened the doors to looking at biomarkers related to inflammatory, metabolic, gastrointestinal uptake/absorption and vascular and endothelial pathways, as well as, tissue biomarkers in skin and the retina.
So, what is a biomarker? The National Cancer Institute defines it as, “a biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease.”3 Biomarkers may be used to track response to treatments to determine if subsequent changes to the treatment plan are needed. For a biomarker to be confirmed, it must be shown to have utility in a least two independent populations.3
Biomarkers for disease management
While risk factors are often used in conjunction with biomarkers (and clinical presentation) in order to diagnose, monitor and manage disease states, it is important to note that they do differ. For example, patient characteristics such as age, weight and smoking history can inform the risk profile for diabetes; whereas HbA1c, a widely accepted biomarker associated with long-term diabetic outcomes, is a measurable biomolecule found within the blood and is indicative of the disease state and treatment management.
Together with the onset of new technologies in proteomics and genomics, combined with high-sensitivity imaging, and high-throughput clinical chemistry/immunoassay tests, the ability to find and validate diabetic biomarkers is increasing. Long-term outcome studies for these biomarkers will also be necessary to determine if the biomarker is a disease predictor or potential treatment target for long-lasting outcome changes. Fortunately, new technologies have provided a pathway for studies to be conducted retroactively on stored patient samples prior to diabetic diagnosis in order to analyze if these samples could have predicted the eventual onset of diabetes in the patients during set time periods from the initial blood draw. Given its manifestation as a complex, multisystem disease, it is important to note, that care must be taken to consider age, weight, BMI, fasting glucose and other potential variables that could alter the biomarker or influence its measurement.
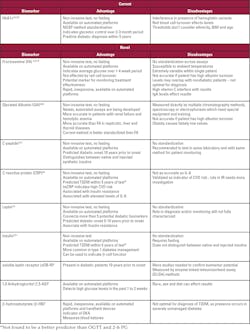
While not all inclusive, Table 1 outlines some novel diabetic biomarkers taken from the overall review, including their advantages and disadvantages. A more detailed summary follows here.
Pros and cons of diabetic biomarkers
C-peptide is typically undetectable or low in type 1 diabetes with an initial decline followed by stabilization, while the marker is normal or high in type 2 diabetes.3,4 Because it is derived in a 1:1 ratio when proinsulin is cleaved to make insulin, C-peptide an intriguing biomarker for insulin production in that high C-peptide levels can indicate high levels of insulin production.7,8
Fructosamine (FA) and glycated albumin (GA) are fairly new markers for early diagnosis of diabetic risk, but remain to be validated.8 FA is created by the glycosylation of serum proteins (~70 percent of which is serum albumin), while GA directly measures the glycosylated albumin; high levels of either indicate high glucose levels over the previous 2-3 weeks. FA levels of > 2.5 mmol/L and GA ≥15.5 percent indicate diabetes, while GA levels of ≥ 13.35 percent indicate prediabetes.6 Trends in FA could be used to determine if treatments are effective or if alterations to treatment plans should be made but it might be unsuitable for diabetes diagnosis.8 Dorcely et al. suggest that combining GA with HbA1c is more sensitive in predicting prediabetes than HbA1c values alone. In addition, 1,5 Anhydroglucitol (1,5 AG) was also identified as a potential biomarker for diabetic treatment monitoring. 1,5 AG absorption is prevented when glucose levels are high, resulting in high urine and low plasma levels of this biomarker in diabetic patients, indicating high glucose levels in the past two weeks.6
Leptin has been identified, in a retrospective study, as a potential “hub” for multiple pathways including C-reactive protein (CRP) binding, glucose homeostasis, adipogenesis and insulin growth factor-binding protein 2 (IGFBP-2) interactions among others, leading to diabetic diagnosis and complications 5-10 years later.4 The role of Leptin as a biomarker needs more controlled studies but provides interesting insight into the interconnected networks leading to T2DM and increased cardiovascular risk, and therefore should be studied as a potential early marker.
Initially, insulin would seem to be an obvious biomarker for diabetes, however, historical insulin results have not led to the accurate prediction of T2DM onset. T2DM is caused primarily by insulin resistance, so the amount of insulin is therefore less relevant than the actual estimate of resistance in a patient, done using the homeostatic model assessment (HOMA).5,7
Dorcely et al. outlined potential novel biomarkers related to insulin resistance such as α-hydroxybutyrate (α-HB), CRP, interlukin-6 (IL-6) and Acylcarnitine.6 α-HB is significantly associated with insulin resistance independent of BMI, sex and age.6 When there is insufficient glucose available for use (either due to endogenously low glucose levels or insulin resistance), the body metabolizes fat into ketones.7 ꞵ-hydroxybutyrate (ꞵ-HB), another diabetic biomarker, is a measure of blood ketones and is likely not a candidate for early detection. Blood ketones consist of, acetoacetate, beta-hydroxybutyrate and acetone and each tests measures one or more of these ketones and is not interchangeable. IL-6 may be a better predictor of T2DM than CRP, as CRP may have a downstream role rather than being casual.6
Scirica’s study on biomarkers, analyzed multiple diverse studies to determine the potential for biomarkers and their clinical implications. In two studies, he observed that high concentrations of NT-proBNP correlated with lower T2DM incidences, noting that in a separate transgenic mouse study, mice overexpressing BNP, were resistant to the effects of a high fat diet.5
Biomarker effficacy advances standardization
Once diagnosed, the need to test patients periodically for biomarkers has tremendous value, including to elucidate if there are changes in the pattern of expression that correlate to disease progression rates and the onset of diabetic complications. Changes in biomarker presentation at time of diagnosis and with disease progression could highlight when changes in treatments are needed.4,5
Further biomarker characterization should be undertaken to provide tools to predict eventual complications such as chronic kidney disease (CKD) and diabetic ketoacidosis (DKA) (both potentially irreversible and life threatening). Risk classification in the future could, and should, include the addition of cardiovascular disease (CVD) risk markers (N-terminal pro b-type natriuretic peptide, high sensitive troponin, high sensitive C-reactive protein), kidney function biomarkers (micro-albumin, creatinine, cystatin C), cholesterol markers (high density lipoproteins, low density lipoproteins, triglycerides, apolipoproteins, lipoprotein(a), ceramide) and liver function biomarkers (alanine aminotransferase, gamma-glutamyl transferase, plasminogen activator inhibitor 1, tissue plasminogen activator, Fetuin A) to further differentiate the risk of diabetic diagnosis and treatment from downstream complications.4,5,6
If validated, certain biomarkers (i.e. FA, GA and 1,5 AG), could provide the physician with enough information to change treatment plans and track efficacy during a 2-3 week period (vs. every 3 months for HbA1c) and then modify treatments if response is not as expected. This would improve the monitoring of T2DM patients to ensure the treatments are effective and that downstream diabetic complications are not progressing well before they manifest clinically. Restricting monitoring to HbA1c, glucose and/or FA or GA levels as indicators of disease progression and regulation limits the knowledge of potentially life threatening implications of T2DM over time. As the diagnosis and monitoring of disease progression increases with the broader adoption of these new biomarkers, the next step will be to advance towards method standardization, much like what was accomplished for HbA1c.
References
- Blaslov K, Naranda F, Kruljac I, Renar I. Treatment approach to type 2 diabetes: Past, present and future. World J Diabetes. 2018;9(12):209-219. doi:10.4239/wjd.v9.i12.209
- DeFronzo R. From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes. 2009;58(4):773-795. doi:10.2337/db09-9028
- Lyons T, Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Translational Research. 2012;159(4):303-312. doi:10.1016/j.trsl.2012.01.009
- Huang T, Glass K, Zeleznik O et al. A Network Analysis of Biomarkers for Type 2 Diabetes. Diabetes. Diabetes. 2019;68:281-290
- Scirica B. Use of Biomarkers in Predicting the Onset, Monitoring the Progression, and Risk Stratification for Patients with Type 2 Diabetes Mellitus. Clin Chem. 2016;63(1):186-195. doi:10.1373/clinchem.2016.255539
- Dorcely B, Katz K, Jagannathan R et al. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2017;Volume 10:345-361. doi:10.2147/dmso.s100074
- Lab Tests Online. https://labtestsonline.org/ Accessed November 14, 2019
- Danese E, Montagnana M, Nouvenne A, Lippi G. Advantages and Pitfalls of Fructosamine and Glycated Albumin in the Diagnosis and Treatment of Diabetes. J Diabetes Sci Technol. 2015;9(2):169-176. doi:10.1177/1932296814567227