Predictive biomarker ratio aids in avoiding high-stakes consequences of severe preeclampsia
With a new predictive preeclampsia biomarker test, laboratories continue to play a critical role with clinicians in improving maternal and newborn mortality in the United States. First cleared by the U.S. Food and Drug Administration in May 2023 for use with hospitalized patients, the sFIt-1/PIGF (soluble fms-like tyrosine kinase-1/placental growth factor) ratio identifies pregnant women at high risk for developing severe preeclampsia, a life-threatening multisystem complication of pregnancy characterized by high blood pressure and signs of organ damage, most commonly to the kidneys and liver.
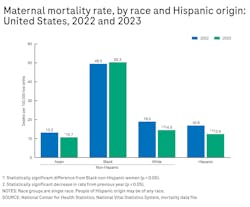
The ratio is considered a significant step forward in the clinical management of a complex, difficult-to-diagnose condition. In the United States, preeclampsia affects 3% to 4% of pregnancies or approximately 165,000 to 220,000 pregnancies each year.1,2,3 Over the past four decades, the condition, for which the only treatment is preterm delivery of the fetus and removal of the placenta, has increased 25% in the United States and is a leading cause of maternal and infant illness and death.4,5 In the United States, preeclampsia disproportionately affects Black women who are more than three times more likely than White women to die from pregnancy-related complications (See Figure 1).6
Value of biomarkers in predicting severe preeclampsia
By definition, preeclampsia is the new onset of hypertension and proteinuria after 20 weeks of gestation, or without proteinuria, new-onset hypertension and any of the following: thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or new onset of headache unresponsive to medication.7 While common patient-reported symptoms of preeclampsia include headaches, visual changes, and epigastric pain,7 the clinical presentation varies widely. Not only does this make diagnosing preeclampsia difficult, it also poses challenges once preeclampsia is present in predicting whether a pregnant woman will progress to severe preeclampsia that could threaten her life or the life of her baby. Preeclampsia can escalate quickly, and the typical indicators of progression, including high blood pressure and protein in the urine, are poor predictors of whether the disease will progress. In fact, these indicators have a positive predictive value of just 18% to 20%.8
Recent studies concluded that identifying patients at high risk of developing severe preeclampsia can lead to better prediction, earlier intervention, and reduced adverse outcomes.9,10 The sFIt-1/PIGF ratio, as a supportive tool for clinical decision-making, is viewed as filling a void in the identification of patients at high risk of developing preeclampsia with severe features.
Specifically, the preeclampsia ratio using biomarkers sFlt-1 and PIGF is intended to help clinicians stratify hospitalized patients into low-and high-risk categories for developing severe preeclampsia within two weeks. sFlt-1 and PlGF are key in the formation of blood vessels during pregnancy, however, an angiogenic imbalance of these biomarkers has been proven to play an important role in the development of preeclampsia. Interestingly, their concentrations in maternal serum are altered even before the onset of the disease, making them a valuable tool for predicting preeclampsia progression.11,12 Nearly 70% of women with a sFlt-1/PlGF ratio greater than 38 delivered their babies within two weeks.10
It's worth noting that in Europe and other countries outside of the United States, sFlt-1 and PIGF biomarkers have been used in predictive testing for severe preeclampsia for more than a decade. As a result, the International Society for the Study of Hypertension in Pregnancy has included the biomarkers in its guidelines.13 Use of the ratio is also part of practice guidelines in the United Kingdom, Canada, Germany, the Czech Republic, Denmark, China, and Spain.
Clinically validated results – the PRAECIS study in the United States, an independent study funded by Cedars-Sinai Medical Center9,10
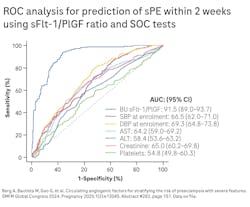
A prospective multicenter trial across 18 U.S. tertiary and community hospitals, the PRAECIS study (Predictive accuracy for serum SFlt-1/PIGF ratio and other standard of care risk biomarkers for development of severe PE study) was conducted to validate the sFlt-1/PIGF ratio for predicting preeclampsia with severe features. The study enrolled pregnant women ≥ 18 years who were admitted to the hospital between 23+0 and 34+6/7 gestational weeks with singleton pregnancies and hypertensive disorders of pregnancy. The primary outcome was the prediction of preeclampsia with severe features within two weeks of testing (See Figure 2). In the study, the sFlt-1/PIGF preeclampsia ratio outperformed the standard of care risk biomarkers that clinicians typically use to predict severe preeclampsia. These include systolic and diastolic blood pressure, alanine aminotransferase, aspartate aminotransferase, creatinine, and platelets.
With a small cohort of 65 patients, a real-world study led by the University of Chicago Medical Center improved upon the PRAECIS results. Published in the American Journal of Obstetrics and Gynecology in July 2024, the study showed a positive predictive value of nearly 79% compared to standard of care. Negative predictive value, similar to the PRAECIS result, was very high at 94%.14 Additionally, this study revealed the rate of preterm delivery was lower in pregnant women with a low sFlt-1/PlGF ratio.
In addition to the health and potentially lifesaving benefits for pregnant mothers and their unborn babies, the swift and accurate prediction of severe preeclampsia has important economic implications. As a leading cause of maternal hospitalizations and preterm deliveries, preeclampsia is a major economic burden on the healthcare system,15 with the majority of these healthcare costs relating to infants born at lower gestational age.16,17 Evaluating and managing preeclampsia requires intensive surveillance, repeated diagnostic testing, and frequent use of both emergency and inpatient services, including neonatal intensive care.18 Categorizing a patient as low risk of developing the condition can reduce healthcare costs by eliminating the need for prolonged hospitalizations, monitoring and related tests.
U.S. clinical practice update on preeclampsia biomarkers
In June 2024, the American College of Obstetricians and Gynecologists (ACOG) issued a clinical practice update acknowledging the sFlt-1/PIGF ratio as an additional tool for use in tandem with other recommended measures to predict risk of developing severe preeclampsia.19 Similar published research and real-world follow-up studies have further supported this update.
“If the sFlt-1:PIGF ratio is used for hospitalized patients admitted between 23 and 35 weeks of gestation with hypertensive disorders, the test is a complementary risk-stratification screen to add to the diagnostic work-up of preeclampsia with severe features.”
ACOG Clinical Practice Update. June 2024.19
Expanding the benefits for future care
Improving maternal and newborn mortality rates and addressing the health disparities that exist in pregnancy-related outcomes are complex issues. As use of the sFlt-1/PIGF preeclampsia ratio becomes more widespread, laboratories will be able to collaborate with clinicians and healthcare systems to ensure pregnant women from all socioeconomic backgrounds have access to this predictive biomarker testing, if the need arises. In addition to predicting life-threatening preeclampsia, promoting earlier intervention and helping to improve maternal, fetal, and neonatal outcomes, the ratio has significant potential in the clinical management of preeclampsia. Its usefulness in the outpatient setting and as an aid in diagnosis would be clinically useful next steps. Some experts believe the biomarker testing may also help catalyze innovation for new preeclampsia therapies. As these possibilities unfold, many women with hypertensive disorders of pregnancy stand to benefit from this much needed and potentially lifesaving biomarker testing.
References
1. Burwick RM, Rodriguez MH. Angiogenic biomarkers in preeclampsia. Obstet Gynecol. 2024;143(4):515-523. doi:10.1097/AOG.0000000000005532.
2. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564. doi:10.1136/bmj.f6564.
3. U.S. pregnancy rates drop during last decade. Cdc.gov. April 12, 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2023/20230412.htm.
4. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008;21(5):521-6. doi:10.1038/ajh.2008.20.
5. Lee R, Brandt JS, Joseph KS, Ananth CV. Pregnancy-associated mortality due to cardiovascular disease: Impact of hypertensive disorders of pregnancy. Paediatr Perinat Epidemiol. 2024;38(3):204-215. doi:10.1111/ppe.13055.
6. Hoyert DL. Maternal mortality rates in the United States, 2023. Cdc.gov. Accessed April 10, 2025. https://www.cdc.gov/nchs/data/hestat/maternal-mortality/2023/Estat-maternal-mortality.pdf.
7. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Obstetrics. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, number 222. Obstet Gynecol. 2020;135(6):e237-e260. doi:10.1097/AOG.0000000000003891.
8. Zhang J, Klebanoff MA, Roberts JM. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstet Gynecol. 2001;97(2):261-7. Doi:10.1016/s0029-7844(00)01125-x.
9. Thadhani R, Lemoine E, Rana S, et al. Circulating angiogenic factor levels in hypertensive disorders of pregnancy. NEJM Evid. 2022;1(12):EVIDoa2200161. doi:10.1056/EVIDoa2200161.
10. Berg A, Bautista M, Guo G, et al. Circulating angiogenic factors for stratifying the risk of preeclampsia with severe features. SMFM Global Congress 2024. Pregnancy 2025;1(2):e12045. Abstract #282, page 157. Data on file.
11. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672-83. doi:10.1056/NEJMoa031884.
12. Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13-22. doi:10.1056/NEJMoa1414838.
13. Magee LA, Brown MA, Hall DR, et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022;27:148-169. doi:10.1016/j.preghy.2021.09.008.
14. Burns LP, Potchileev S, Mueller A, et al. Real-world evidence for the utility of serum soluble fms-like tyrosine kinase 1/placental growth factor test for routine clinical evaluation of hospitalized women with hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2025;232(4):385.e1-385.e21. doi:10.1016/j.ajog.2024.07.015.
15. Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365. doi:10.1155/2011/214365.
16. Hao J, Hassen D, Hao Q, et al. Maternal and infant health care costs related to preeclampsia. Obstet Gynecol. 2019;134(6):1227-1233. doi:10.1097/AOG.0000000000003581.
17. Stevens W, Shih T, Incerti D, et al. Short-term costs of preeclampsia to the United States health care system. Am J Obstet Gynecol. 2017;217(3):237-248.e16. doi:10.1016/j.ajog.2017.04.032.
18. Shih T, Peneva D, Xu X, et al. The rising burden of preeclampsia in the United States impacts both maternal and child health. Am J Perinatol. 2016;33(4):329-38. doi:10.1055/s-0035-1564881.
19. Biomarker prediction of preeclampsia with severe features. Obstet Gynecol. 2024. doi:10.1097/AOG.0000000000005576.