Immunohistochemistry (IHC) was described in 1941,1 but it was not until the early 1970s that anatomic pathology used labeled antibodies on formalin-fixed, paraffin-embedded tissues for routine diagnostics.2-4 The development of the IHC method during the 40 years since has brought improvements such as monoclonal antibodies to human antigens,5 antigen retrieval,6 automation,7-9 and polymer-based visualization systems.10 The importance of improving the standardization of IHC11 has become even more apparent following the discovery of high numbers of false negative and false positive results in IHC determinations of estrogen receptor.12
With more than 40 years of continued improvement of reagents, manufacturing, instruments and analysis, one would expect that IHC-based cancer diagnostics would by now be a completely routine and automatic procedure in which a tissue sample is just sent through the instrument, with a correct result emerging every single time. Yet, data from more than 26,000 slides reveal that nearly one out of three slides does not give a satisfactory staining result.13 Incorrect results not only increase the risk of misdiagnosis affecting the individual patient but also have an economic impact: every dollar that laboratories spend by using low-quality reagents with unvalidated test protocols could potentially increase the economic burden on the healthcare system.14
IHC as a qualitative test
In cancer diagnostics, class I in vitro diagnostic antibodies are used for identification of proteins in or on cells to classify a tumor. The classification involves a panel of antibodies where each antibody provides information about the type of tumor, and the combined information from all of the staining results will give a clear picture of the tumor type. The IHC staining used for classification is qualitative in nature, in that each result answers the question, “Is this protein present in the tissue or not?” With the exception of a few class II and class III predictive and prognostic markers such as ER, PR, and HER2 that are available as semi-quantitative assays, the qualitative IHC results simply, but accurately, aid the pathologist in classifying the tumor. However, because IHC has for decades been lacking standardization of test performance characteristics, IHC has been perceived more as an art form than a laboratory test such as ELISA.15 A number of inherent physical and chemical properties of IHC influence the perceived staining intensity, so the mindset when doing qualitative IHC should only be to ensure correct positive or negative answers, not to determine how much protein is in the specimen.
Why IHC is not quantitative
IHC—using diaminobenzidine (DAB) as chromagen—has some limitations as a quantitative method. Some of the listed limitations affect the perceived staining intensity of any chromogen, not only DAB.
- The brown DAB end reaction product is not a true absorber of light, but rather it scatters light.16 In other words, the more protein there is in the tissue, the more DAB will precipitate, but the color does not follow a linear relationship between the amount of DAB and how dark it looks.
- Amplification steps in visualization systems convert the antigen/antibody complex into a visual signal in a non-linear fashion,15 meaning that if one protein molecule results in one DAB molecule being precipitated, it does not follow that 10 protein molecules cause 10 DAB molecules to be precipitated.
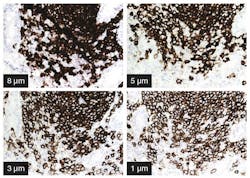
- Thick sections will tend to give stronger staining intensity than thin sections (Figure 1). This is simply because there is more tissue with more antigen into which the DAB end-product may precipitate.
- Pre-analytical factors such as fixation delay and fixation time cannot be kept constant for all samples at all times.
- Microscopic evaluation of staining intensity is a subjective estimate by individual observers with different training using different microscopes with varying light sources and filters. (Digital imaging with scoring algorithm software is not standard equipment.)
- Counterstaining is almost always the blue hematoxylin coloring of nuclei. The intensity of the contrast color will affect perceived intensity of the antigen staining, especially for nuclear antigens.
- The dynamic range of IHC is about one to two logs,17,18 but protein expression can vary up to four logs,18 corresponding to 200 to 2,000,000 molecules per cell. The IHC method will thus not reflect changes in staining intensity outside the dynamic range.
Why do qualitative IHC?
So why do cancer diagnostics rely so heavily on what seems like a poor quantitative method for protein detection in tissue? The reason is that IHC can very specifically detect proteins in tissue if properly conducted with specific and sensitive antibodies, high-quality reagents, and calibrated protocols. When correctly calibrated, IHC is also capable of displaying varying levels of expression from negative to weak/moderate and moderate/strong. That is not the same as the staining intensity being a linear, quantitative method for protein amount. However, when the objective of most IHC tests for cancer diagnostics is to demonstrate the presence or absence of a given protein, the IHC test is first and foremost qualitative.
So the answer to “Why do qualitative IHC?” is that it is an excellent and specific method for qualitative protein detection for the identification of cell types using specific markers and therefore for the classification of tumors. Correct tumor classification is imperative for the oncologist’s treatment decision.
Quality is in the controls
To accurately provide qualitative answers, careful calibration of assays is needed to ensure that each test has the correct specificity and sensitivity to detect the protein in the tissue. An international committee of IHC proficiency testing experts has introduced practical guidelines and standards that can be adopted for selection of human tissues for use as daily controls in pathology laboratories.19,20 Among the many efforts to improve standardization in IHC, it is highly recommended to always use on-slide control tissues that show low levels of expression of the protein in a consistent and predictable manner.
The best control is internal tissue elements within the patient sample showing high, low, and no expression, but all three categories of internal controls are rarely present within the patient sample. Thus, including on-slide control tissue that has been subjected to similar pre-treatment processes will increase the chance to detect otherwise false negative or false positive samples. The on-slide tissue controls should contain structures displaying staining intensity in the lower part of the staining intensity range in order to reduce the risk of false negatives. The controls should also contain tissue structures with known high expression of the protein, to confirm that the protocol does not stain to a level that would compromise diagnosis. Finally, negative structures ensure no false positivity.
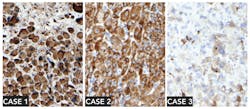
Why go through all the trouble setting up on-slide control tissue for every protocol? The reason is that tumor cells display abnormal staining patterns by nature (Figure 2), and therefore both high-expression and low-expression patterns should be expected. A qualitative IHC test should be able to correctly indicate if the marker is present, even in a low amount. The risk of a false positive is much lower than the risk of a false negative, since up to 90 percent of insufficient scores in an external quality scheme are due to a false negative result.13
A greater focus on appropriate tissue controls, i.e., high expressors (HE), low expressors (LE) and no expressors (NE), is essential for achieving a correct test result. The staining protocol should be calibrated to create a visual positive reaction at or above the lower detection limit defined by structures in the tissue that are known to have low expression of the marker. Such structures are exemplified in both literature and material from vendors of in vitro diagnostic antibodies.19,21
Incorporating controls in daily routine
Many pathology labs use batch-run controls with the control section on a separate slide to indicate that the staining process has run correctly. However, the most appropriate IHC controls, when internal controls do not contain HE, LE, and NE structures, are on-slide controls (Figure 3). The advantage is that the control tissue will receive identical reagents and incubations as the patient sample because they are on the same glass slide.
Conclusion
In cancer diagnostics, IHC tests are mostly used as a qualitative tool to confirm or reject the presence of a protein. The collective information from a panel of antibodies helps to classify the tumor and thereby provide a correct diagnosis. It is the pathologist’s report on the collective findings that aid the oncologist in determining the best possible treatment for the cancer patient. Carefully calibrated assays, combined with on-slide tissue controls that should contain tissue structures with no, low, and high expression of the target protein, are the best way to achieve confidence in the IHC staining result. The mindset when doing qualitative IHC should be to ensure correctness and robustness of all markers for all patients, including those patients whose tumors express low levels of the antigen.
References
- Coons AH, Creech HJ, Jones RN. Immunological properties of an antibody containing a fluorescent group. Experimental Biology and Medicine. June 1, 1941;47(2):200-202.
- Taylor CR. The nature of Reed-Sternberg cells and other malignant “reticulum” cells. Lancet. Oct. 5 1974;2(7884):802-807.
- Taylor CR, Burns J. The demonstration of plasma cells and other immunoglobulin-containing cells in formalin-fixed, paraffin-embedded tissues using peroxidase-labelled antibody. J Clin Pathol. 1974;27(1):14-20.
- Taylor CR, Mason DY. The immunohistological detection of intracellular immunoglobulin in formalin-paraffin sections from multiple myeloma and related conditions using the immunoperoxidase technique. Clinical and Experimental Immunology. 1974;18(3):417-429.
- McMichael AJ, Pilch JR, Galfre G, Mason DY, Fabre JW, Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. European Journal of Immunology. 1979;9(3):205-210.
- Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 1991;39(6):741-748.
- Stark E, Faltinat D, Von der Fecht R. An automated device for immunocytochemistry. Journal of Immunological Methods. 1988;107(1):89-92.
- Tubbs RR, Bauer TW. Automation of immunohistology. Archives of Pathology & Laboratory Medicine. 1989;113(6):653-657.
- MaWhinney WH, Warford A, Rae MJ, Lauder I. Automated immunochemistry. J Clin Pathol. 1990;43(7):591-596.
- Sabattini E, Bisgaard K, Ascani S, et al. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51(7):506-511.
- Taylor CR. An exaltation of experts: concerted efforts in the standardization of immunohistochemistry. Appl Immunohistochem. 1993;1:232-243.
- Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Journal of Oncology Practice / American Society of Clinical Oncology. 2010;6(4):195-197.
- Nielsen S. External quality assessment for immunohistochemistry: experiences from NordiQC. Biotechnic & Histochemistry: Official Publication of the Biological Stain Commission. 2015;90(5):331-340.
- Vyberg M, Nielsen S, Roge R, et al. Immunohistochemical expression of HER2 in breast cancer: socioeconomic impact of inaccurate tests. BMC Health Services Research. 2015;15(1):352.
- Torlakovic EE, Nielsen S, Vyberg M, Taylor CR. Getting controls under control: the time is now for immunohistochemistry. J Clin Pathol. Aug 18, 2015.
- van der Loos CM. Multiple immunoenzyme staining: methods and visualizations for the observation with spectral imaging. Journal of Histochemistry and Cytochemistry. 11/19/received. 11/26/accepted. 2008;56(4):313-328.
- Lohse J, Petersen KH, Woller NC, Pedersen HC, Skladtchikova G,
Jørgensen RM. Improved catalyzed reporter deposition, iCARD. Bioconjugate Chemistry. 2014/06/18 2014;25(6):1036-1042. - Rimm DL. What brown cannot do for you. Nat Biotech. 2006;24(8):914-916.
- Torlakovic EE, Nielsen S, Francis G, et al. Standardization of positive controls in diagnostic immunohistochemistry: recommendations from the international ad hoc expert committee. Applied Immunohistochemistry & Molecular Morphology. 2015;23(1):1-18.
- Torlakovic EE, Francis G, Garratt J, et al. Standardization of negative controls in diagnostic immunohistochemistry: recommendations from the international ad hoc expert panel. Applied Immunohistochemistry & Molecular Morphology: AIMM/official publication of the Society for Applied Immunohistochemistry. 2014;22(4):241-252.
- Company. DAAT. Dako Atlas of Stains, 4th Ed. 2013.