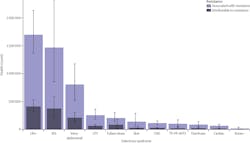
In order to combat this growing threat, the global healthcare community is researching a number of both drug-based and non-drug-based solutions. Drug-based approaches pose a number of immediate and long-term challenges. The World Health Organization’s (WHO) report titled “2021 Antibacterial agents in clinical and preclinical development: an overview and analysis” characterizes the current number of antibacterial drugs in preclinical and clinical development as stagnant and far from meeting global needs.3 Further, the same WHO analysis showed that in 2021 there were only 27 new antibiotics in clinical development against priority pathogens, compared to 31 products in 2017.
Lower respiratory tract infections and AMR
Lower respiratory tract infections (LRTIs) such as pneumonia pose a particular threat to antimicrobial resistance for a number of reasons. First, these infections are highly prevalent in global populations. In 2015, it was estimated that LRTIs caused 2.74 million deaths worldwide.4 High rates of antimicrobial resistance have been observed for the pathogens responsible for LRTIs.5 In a 2021 study, researchers processed a total of 7,038 samples of sputum and bronchial aspirate according to the standard microbiological methods. In these samples a “very high rate of resistance” (98–100%) was observed among Acinetobacter baumannii isolates to Amoxicillin/Clavulanic acid, Cefotaxime, Ciprofloxacin, Ertapenem, Gentamicin, Imipenem, and Trimethoprim/Sulfamethoxazole.5
Where COVID-19 has already exacerbated concerns around AMR more generally,2 the virus’ targeting of the respiratory tract, particularly in its earlier mutations, only adds to the types of respiratory symptoms being treated with inappropriate antibiotics. The high rates of antimicrobial resistance are not really surprising due to how similarly many LRTIs present and the common approach of prescribing broad spectrum antibiotics.
Pneumonia, one of many LRTIs raising concerns, is among the most common reasons for inpatient antibiotic use and overuse.6 Hospital-associated pneumonia (HAP) accounts for 22% of all nosocomial infections and ventilator-associated pneumonia (VAP) mortality rates range from 20% to 60%.7
As such, a number of recent studies have examined best practices for antibiotic stewardship when it comes to lower respiratory tract infections and found inappropriate initial antimicrobial therapy is associated with increased mortality in patients with pneumonia.
Historically, providers prescribed long durations of antibiotics for pneumonia because of concerns that short courses could lead to disease relapse or progression.8 Recent studies, including multiple randomized controlled trials and systematic reviews, have demonstrated that shorter antibiotic therapy is safe and equally effective for most patients with pneumonia, avoiding longer antibiotic treatment that puts patients at risk for antibiotic-associated adverse events, C. difficile infection, and multi-drug resistant organisms (MDROs).8 That same study found that more than two thirds (67.8%, 4,391/6,481) of patients received excess antibiotic therapy duration, largely due to excessive prescribing at discharge.8
A study7 published in 2021 assessing antibiotic de-escalation in patients with nosocomial pneumonia showed that more de-escalations occurred when diagnostic tests were ordered; and importantly, in these patients de-escalation was associated with fewer antibiotic days (mean 9 vs. 11), reduced episodes of C. diff infection (2.2% vs. 3.8%) and shorter hospital days (mean 20 vs. 22 days), shorter ICU stays, less time on ventilator, reduced acute kidney injury (AKI) and reduced initiation of renal replacement therapy.7 Moreover, there was no difference in in-hospital mortality, 14-day all-cause mortality, readmission for any indication, or treatment re-escalation in patients who received de-escalation versus no de-escalation
These studies demonstrate that excess antibiotic treatment is not associated with lower rates of any adverse outcomes (that is, death, readmission, emergency department visit, or C. diff infection). In fact, each excess day of antibiotic therapy is associated with 5% increased odds of experiencing an antibiotic-associated adverse event, and an estimated 1.03-fold increase in the odds of AMR associated with each additional day of antibiotics.
Testing methodologies
For LRTIs and other infections, microbiological cultures are widely used as the standard of care for identifying the presence of pathogens, and empirical broad-spectrum antibiotic therapy is initiated while waiting for the results. Limitations of microbiological cultures are well-acknowledged. These limitations are attributable to results taking several days, as well as factors such as dependence on microbial growth, growth being affected by sample transport time and temperature, or being inhibited by prior antibiotic treatment, contributing to sensitivity challenges. These limitations further confound the diagnostic picture in patients undergoing a long-term hospital or ICU stay for whom clinicians usually order subsequent cultures at multiple intervals throughout a patient’s stay.
Alternative testing approaches can support antibiotic stewardship and limit the use of broad-spectrum antibiotics. Multiplex molecular diagnostic panels offer a rapid and complementary approach for identifying pathogens and AMR markers. Yet many question if these panels are appropriately sensitive and suitable for reliable, accurate testing.
A recent study9 examined serial microbiological culture samples taken from hospitalized COVID pneumonia patients by comparing the results of a culture to a multiplex PCR lower respiratory panel for detection of pathogens from serial specimens collected from the same patient. Serial specimen analysis demonstrated that the multiplex Unyvero PCR panel was not only as accurate at detecting a pathogen, but in some cases, even more precise. Additional pathogens detected by the PCR panel could be confirmed in many instances by culture positivity for the same organism in another sample obtained from the same patient. This publication highlights the ability of the multiplex lower respiratory panel in detecting potential pneumonia pathogens earlier than culture or very early during an infection.
Another study5 was conducted with patients who were admitted to the hospital with suspected pneumonia, had a clinical indication for bronchoscopy with bronchoalveolar lavage, and were at risk of Gram-negative bacterial infection. This study found that using a comprehensive multiplex molecular lower respiratory panel such as the Unyvero pneumonia test shortened inappropriate antibiotic therapy duration by 39 hours (p<0.0001), and reduced overall antibiotic therapy duration by 34 hours (22.5%). The multiplex panel also reduced the use of inappropriate antibiotic therapy by 45% (p<0.0001). In addition, patients tested with the molecular multiplex panel had a three-times higher probability of receiving appropriate antibiotic therapy.
Looking ahead
It is clear antimicrobial resistance in lower respiratory tract infections poses a significant threat to the global population. The global health community needs to continue embracing non-drug- based solutions in order to outpace growing AMR. Multiple studies have demonstrated the accuracy and impact of multiplex molecular testing. As clinicians continue to grow in confidence around the validity of multiplex PCR panels, we can develop a clearer path forward in combatting antimicrobial resistance.
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;12;399(10325):629-655. doi: 10.1016/S0140-6736(21)02724-0.
- COVID-19 reverses progress in fight against antimicrobial resistance in U.S. Centers for Disease Control and Prevention. Published July 12, 2022. Accessed November 2, 2022. https://www.cdc.gov/media/releases/2022/s0712-Antimicrobial-Resistance.html.
- 2021 antibacterial agents in clinical and preclinical development: An overview and analysis. World Health Organization. Published May 27, 2022. Accessed November 2, 2022. https://www.who.int/publications/i/item/9789240047655.
- GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(11):1133-1161. doi: 10.1016/S1473-3099(17)30396-1.
- Santella B, Serretiello E, De Filippis A, et al. Lower respiratory tract pathogens and their antimicrobial susceptibility pattern: A 5-year study. Antibiotics (Basel). 2021;13;10(7):851. doi: 10.3390/antibiotics10070851.
- Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200.
- Ilges D, Ritchie DJ, Krekel T, et al. Assessment of antibiotic de-escalation by spectrum score in patients with nosocomial pneumonia: A single-center, retrospective cohort study. Open Forum Infect Dis. 2021;8(11):ofab508. doi: 10.1093/ofid/ofab508.
- Vaughn VM, Flanders SA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019;171(3):153-163. doi:10.7326/M18-3640.
- Tellapragada C, Ydsten KA, Ternhag A, Giske CG. Evaluation of a pneumonia multiplex PCR panel for detection of bacterial respiratory tract pathogens from serial specimens collected from hospitalized COVID-19 patients. Eur J Clin Microbiol Infect Dis. 2022;41(7):1093-1098. doi: 10.1007/s10096-022-04466-9.