The 2024 Medical Laboratory Observer (MLO) State of the Industry (SOI) survey on molecular diagnostics (MDx) queried medical laboratory professionals on MDx testing trends, including types of testing modalities used in their labs, methods for handling quality issues, and volume of respiratory testing compared with last year.
More than four years out from the height of the COVID-19 pandemic, we eliminated COVID-19 specific testing questions, although we continued to ask lab professionals about supply shortages given continued disruptions in the healthcare supply chain.
New for this year, the survey questioned lab professionals about their development of tools/processes to manage decreased access to laboratory developed tests (LDT). This topic is top of mind for many medical laboratorians given the U.S. Food and Drug Administration’s (FDA) final rule issued on April 29, 2024, which made explicit that:
“in vitro diagnostic products (IVDs) are devices under the Federal Food, Drug, and Cosmetic Act (FD&C Act) including when the manufacturer of the IVD is a laboratory.”1
Key 2024 survey findings:
· Testing modalities: A reported 5% increase in DNA/genetic testing compared with last year (20% in 2024, up from 15% 2023), while reverse transcriptase quantitative polymerase chain reaction (rRT-qPCR) once again topped the list of MDx modalities employed (64% of respondents).
· Supply chain issues: The percentage of lab professionals reporting supply related testing disruptions dropped again this year at 38% (down from 53% in 2023, and 85% in 2022).
· Quality assurance measures: There was a jump in several reported actions labs take to reduce the number of potential false positive test results, including:
o A 46% increase in those reporting to verify all pre-analysis steps are performed correctly (68% in 2024, up from 22% in 2023).
o A 33% increase in those reporting to repeat the test with the same sample and new extractions (47% in 2024, up from 14% in 2023).
o A 26% increase in both those reporting to refer to quality assurance program guidance (34% in 2024, up from 7% in 2023) and those reporting to decontaminate the work/test area per laboratory procedures (59% in 2024, up from 33% 2023).
Nearly 100 lab professionals participated in the survey. Demographics changed little since last year, with most respondents working in Lab Manager, Administrator, Supervisor, or Lab Director positions (60%) employed by hospital/health system labs (66%).
More than half of those surveyed work in labs that perform 100 or fewer molecular-based tests (non-COVID-19) annually (58%), while nearly one-quarter (22%) are in labs that perform 401+ MDX tests per year. Of the remaining respondents, annual lab volumes are as followed:
· 101-200 tests: 13%
· 201-300 tests: 5%
· 301-400 tests: 1%
Alongside the quantitative survey results data, we include commentary from medical laboratory professionals and suppliers in the MDx space.
MDx testing modality trends
When asked what types of MDx tests they use in their laboratories, reverse transcriptase quantitative polymerase chain reaction (rRT-qPCR) ranked highest, with 63% of respondents reporting its usage (62% in 2023).
Nearly half of respondents reported their labs’ usage of rapid molecular tests, down 10% since last year (49% in 2024, down from 59% in 2023). Close to half of those surveyed reported using rapid antigen tests, which was up 6% compared with last year (49% in 2024, up from 43% 2023).
There was a 5% increase in those reporting use of DNA/genetic testing (20% in 2024, up from 15% 2023), and a 3% increase in those with labs using flow cytometry (21% in 2024 up from 18% 2023).
Fewer medical lab professionals reported using:
· Reverse transcription loop-mediated isothermal amplification (RT-LAMP): 9% in 2024 (down from 12% 2023)
· Recombinase polymerase amplification (RPA): 4% in 2024 (down from 9% in 2023)
· CRISPR-based diagnostics: 0% in 2024 (down from 2% in 2023)
Reported usage of liquid biopsy and next generation sequencing was on par with last year’s survey results, with 6% reporting use of the former (5% in 2023) and 18% the latter (18% in 2023).
New for this year, we asked medical laboratory professionals about their use of mass spectrometry testing, with 8% reporting its usage in their labs.
Among the 11% of survey respondents selecting “other,” cited MDx modalities included:
· Reverse transcription-polymerase chain reaction (RT-PCR)
· Nucleic acid amplification test (NAAT)
· Thrombotic microangiopathy (TMA)
· Transferrin receptor testing (TFR)
· Indirect haemagglutination assay (IHA)
· Food intolerance
“We are moving away from traditional biochemical testing which, as a reference lab, have used for confirmation testing for rapid tests,” said Donna Ferguson, PHM, PhD, Public Health Laboratory Director, Monterey County Public Health Laboratory in Salinas, CA. “We are better equipped to test for outbreaks, and God forbid, future pandemics. We’ve purchased instruments for high throughput PCR testing including thermocyclers and instruments for extraction.”
“We have also implemented whole genome sequencing but are using it for non-diagnostic testing (i.e. surveillance),” Ferguson added. “We recently implemented diagnostic testing using the Bruker MALDI and purchased a ddPCR for both diagnostic and environmental testing.”
Lee Panton, former Lab Director from California who now serves as a consultant, commented on the pros and cons of various MDx instrument sizing, stating:
“The new smaller MDx instruments are easier to use and feature a growing number of tests; therefore, more labs can find the space and personnel to run them. These often offer tests that are CLIA waived so RNs can run them. Larger instruments are coming up with a bigger variety of tests, but low volumes in small and medium sized labs may not make it cost effective.”
Mary Jo Blooflat, MT(ASCP), Director of Clinical Laboratory Services, Northfield Hospital, Northfield, MN, commented on the growing number of MDx tests for women’s health, noting how her lab has recently began using a vaginitis panel.
“We’ve had the chlamydia and gonorrhoeae panel for quite a while, but I would like to see something for syphilis given we have to test all pregnant women twice for it during their pregnancies, during an early visit, and after they have delivered their babies,” she added.
“In critical access hospitals, I am seeing an increased use of Cepheid PCR technology,” said Steve Raymond, MLS, MS, MPA, Interim Lab Director, Northeast Montana Health Services Laboratory.
Nathan Patton, Vice President of Marketing for Near Patient Care at Roche Diagnostics commented on several key trends that are shaping the rapidly evolving MDx testing landscape, most of which include bringing testing closer to the patient.
“With the emergence of new technologies, decentralized healthcare sites such as urgent care centers, physician offices, and emergency rooms now have the opportunity to bring testing in-house that they may have previously sent out or managed via centralized laboratories. This shift to molecular point-of-care testing can provide advantages to clinicians and patients. It includes increased access to gold-standard PCR testing and reduced turnaround times, enabling clinicians to make accurate diagnoses and promptly determine the appropriate treatment."
“Advancements in PCR-based diagnostics have enabled providers to test for multiple pathogens in a single multiplex assay and deliver results in 15 to 20 minutes, increasing these diagnostics’ applicability in POC settings,” Patton added. “Patients can also benefit from receiving their results during their office visit and leave with a prescription or a clear direction on their treatment plan.”
According to Jackie Weiss, PhD, Scientific Affairs Liaison at EUROIMMUN US (part of Revvity), molecular testing will continue to be a valuable tool in the fight against antimicrobial resistance (AMR). She stated:
“While efforts to curb AMR often focus on antibiotic resistance, the burden of antifungal resistances continues to increase. Historically, culture-based methods have been used to assess antifungal susceptibility. However, molecular techniques, such as PCR, produce results up to four weeks earlier than culture. In the case of invasive fungal infections, time is of the essence, as delays in appropriate treatment are linked to increased mortality.”
“There is a growing trend towards the development and validation of multiplex PCR assays that can rapidly identify the most resistance-associated mutations in clinically relevant fungal species,” Dr. Weiss continued. “This is particularly important given the limited number of antifungal drug classes available and the rising incidence of antifungal-resistant infections.”
Respiratory testing trends
When asked how much their respiratory testing has increased in 2024 compared with 2023, nearly half reported a 0-25% increase (49%) and one-fifth (20%) a 26-50% increase. Additionally, 17% noted their respiratory testing had decreased since last year.
The remaining responses are as follows:
- 6% reported a 51-75% increase
- 7% reported a 76-100% increase
- 0% reported a 101+% increase
Raymond reported his lab is seeing an increase in respiratory testing, as well as C. diff testing.
Commenting on MDx testing trends in respiratory and other areas, Panton stated:
“I am excited about the new testing for MTB and STDs. Speeding these up will make a big improvement in care. The GI tests and wide panels for respiratory disease are very good. Subacute units for respirator dependent patients benefit with these complete test menus. When they get the sniffles, we need to test them for serious illness versus a cold quickly. Patients coming to the ER want to know what they have specifically. Another area with a major need is testing for sepsis quickly. MDx will make major improvements in survival, LOS, and antibiotic stewardship.”
According to Patton, there is an increased demand for prompt detection of pathogens like SARS-CoV-2, influenza A/B and RSV nucleic acid test, as well as tests for sexually transmitted infections (STI).
“Introducing multiplex PCR diagnostic tests with quick turnaround times into near-patient care environments such as emergency departments, urgent care facilities, and physician offices has the potential to provide swift results without sacrificing precision, expediting clinical decision-making processes,” he stated. “This approach can help reduce unnecessary antibiotic usage, facilitate targeted treatment strategies, and ultimately enhance patient outcomes and healthcare system efficiency.”2-7
Access to laboratory developed tests (LDT)
The FDA’s final rule to claiming regulatory authority over laboratory developed tests (LDT) has been met with concerns in the medical lab community and beyond. As reported by the American Society for Clinical Pathology (ASCP), “laboratories with new LDTs should expect a far more laborious, time consuming, and expensive FDA-approval process”8
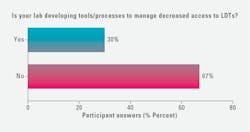
When asked if their labs were developing tools/processes to manage decreased access to LDTs, 30% of survey respondents selected “yes,” 67% selected “no,” and 9% selected “other” with write in comments including:
· [We are working to] determine if any of our lab developed tests fall under any category of continuing enforcement discretion. [We are reviewing our] budget, looking for alternate funding sources, developing and implementing systems and processes to comply [with at] least Stage 1.
· We have created a team to work on LDT compliance but no plan regarding decreased access yet.
· We are sending [LDTs] to [our] sister laboratory.
“As a public health laboratory, we still rely heavily on LDTs,” Ferguson commented. “We purchased two liquid handlers this past year to automate procedures that we were performing manually. We stay abreast of recent developments via webinars and Association of Public Health Laboratories (APHL). We plan on keeping our current LDTs and to go through the FDA approval process if necessary.”
George Manolopoulos, Clinical Lab Manager, Pathology Reference Lab, San Antonio, noted how his lab had “discontinued all LDTs way before the new LDT ruling and replaced them with FDA approved tests.” He added, “we will definitely think twice before deciding to bring an LDT on board in the future.”
Panton noted several questions medical lab professionals have on their minds in response to the FDA action on LDTs: “One question we all have is, for example, if the product insert says to use serum, can we also use plasma? Can we still validate it with a number of patient parallel samples? Such as collect 20 patient samples and test both? If the results are the same, can we modify? Is ANY modification prohibited?”
Robert Cardwell, Chief Strategy Officer at the Genetics Institute of America (GIA), has been closely monitoring the impact of the FDA’s ruling on LDTs. He noted, “While many virology and microbiology assays are already available as FDA-cleared kits, the vast majority of human DNA and RNA studies remain laboratory-developed tests.”
“This has raised significant concerns within the community,” Cardwell explained. “The assays that genetics and genomics laboratories rely on for personalized medicine - such as those used to characterize a patient’s tumor and identify response to therapeutics - are now subject to FDA review and approval. Pursuing regulatory clearance through either a PMA or a de novo application involves substantial costs.”
Cardwell added, “In the molecular diagnostic space, it’s an unusual position for me to say this, but innovation and care in other parts of the world may soon surpass what we have in the United States.”
Discussing GIA’s strategy in light of the FDA ruling, Cardwell highlighted how they have a robust pipeline for assay development and are continually expanding their test menu.
“We’ve reprioritized our molecular diagnostic development,” he stated. “Assays that are already FDA-cleared have been moved to the forefront of our pipeline. However, we will continue to develop appropriate LDTs.”
“With the new ruling, I believe the FDA is setting a baseline for quality with LDTs,” said Steven Kelly, Technical Product Manager, Streck. “There has been a lot of pushback, especially from smaller labs that might not have the resources to comply with the regulations. What I fear is consolidation where smaller university labs might start selling their LDTs to a large commercial lab, which leads to less competition. This could, in turn, result in higher costs for these tests, and CMS and private insurers will always try to avoid overpaying for anything.”
“There is a need for third party proficiency or third-party quality control that can help these labs prove to the FDA that their staff are proficient and their LDTs are accurate and effective,” he added.
Quality control/assurance trends
The survey questioned lab professionals about their quality control measures, asking how they handle questionable results with MDx tests. Those reporting they repeat the test has continued trending downward (45% in 2024 down from 55% in 2023 and 65% in 2022).
Conversely, lab professionals who report verifying all pre-analytical, analytical, and/or post-analytical steps are performed correctly has continued trending upward (24% in 2024, up from 15% in 2023 and 7% in 2022).
Additionally, 15% reported repeating the test with a different employee/equipment/test, which was not an answer option in the 2023 survey.
Those reporting their lab send results to another lab for verification and second test continued a downward trend (6% in 2024, 8% in 2023, 20% in 2022).
Among those who reported using “other” when handling questionable results with MDx tests, write-in responses included:
· Repeat the test, [verify] all steps, and/or send-out if results still questionable.
· Verify all pre-analytical, analytical, and post-analytical steps are performed correctly and repeat the test.
· Verify pre-analytical, analytical, and post-analytical steps, QC etc., then retest sample.
· Repeat the test and then send to our internal and external reference lab as they have other methods to verify testing.
· Notify administrators.
· Analyze QC with company, retest, correlate with NGS.
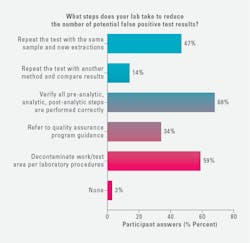
This year, more medical lab professionals reported taking the following actions to reduce the number of potential false positive test results:
· 68% verify all pre-analysis steps are performed correctly, a 46% increase over 2023.
· 59% decontaminate the work/test area per laboratory procedures, up 26% from 2023.
· 47% repeat the test with the same sample and new extractions, a 33% increase over 2023.
· 34% refer to quality assurance program guidance, up 27% from 2023.
Those survey respondents reporting to repeat the test with another method and compare the results held steady, at 14% in 2024, compared with 15% in 2023.
When asked if they use another step to reduce the number of potential false positive test results not listed above, survey respondents reported:
· Decontaminating after each test with bleach
· Performing contamination checks
· Performing wipe test of work area to confirm no contamination
Kelly spoke on the topic of MDx testing quality control and assurance as it relates to automation and lab tech shortages stating:
“With today’s MDx test platforms, the technician can simply place the sample and the platform will automatically do the extraction, purification, hybridization, or PCR, and then spit out the results. It’s the right time for this automation given the deficit in medical technologists and clinical lab scientists because it helps fill these talent gaps.”
On the other hand, Kelly points out how with automation, technicians don’t manually test each step for accuracy.
“Before these automated platforms, we had to do all those steps by hand, and we had to manually control for each step. For example, I could look at the amplification curve or the electropherogram of the sequencing and could tell if there's garbage down at the bottom that showed it wasn't a really clean sample. With automation, these platforms are more of a black box where the technician has to have faith that it is functioning as intended.”
Kelly added: “While the technicians running these machines can be generalists, which is positive given the shortages of techs with more advanced skills, it is still important that they how know a lab functions, the purpose of the lab test, etc. That way, the machine can do the heavy lifting, but the technician can tick off all the boxes to ensure they are doing everything according to their CAP checklist.”
Commenting on this year’s survey results, where more respondents report taking steps to reduce the number of potential false positive results (e.g., repeating the test, decontaminating the work area), Kelly believes this reflects a growing focus on quality control with MDx testing.
“Quality control to check work at each step is where we need to focus,” said Kelly. “So, let’s have our quality control systems take care of some of that heavy lifting of assuring the test is accurate and provides good data for treatment options. That’s what Streck provides. Our products evaluate the accuracy and precision of instruments and techniques to give lab teams confidence in the validity of their results.”
Supply chain trends
While there have been recent reports of supply shortages in various categories, most recently blood culture vials, the medical lab professionals who took part in this survey reported fewer issues with maintaining a supply of testing products due to supply chain issues compared with previous years’ responses.
When asked if a lack of sample-related products has impeded their testing capacity at times, 38% selected “yes,” down from 53% in 2023, and 85% in 2022. Conversely, 62% chose “no” indicating they have adequate testing supplies to meet testing demands, up from 47% in 2023, and 15% in 2022.
“Supplies and reagents have been arriving on time and in full quantities for the most part,” said Manolopoulos. “Only the occasional back orders on plastics but on items that we only order once every few years, not on our regular ancillary supplies.”
While the number of medical lab professionals reporting supply chain issues has trended downward, it is worth noting that nearly two-fifths still report some level of supply disruptions impacting their work.
When asked which testing supplies they are having trouble sourcing due to supply chain issues:
· 27% selected controls/reagents
· 26% blood collection tubes
· 21% culture media
· 16% lab plastics not otherwise noted
· 13% winged blood collection sets
· 11% pipettes
· 11% testing kits for SARS-CoV-2
· 10% swabs/consumables
· 10% transport media
· 8% blood culture transfer devices
· 7% PPE
· 3% urine testing supplies
More than one-third of survey respondents (36%) reported no issue sourcing these supplies. Among the 4% of respondents who chose “other,” several note their trouble sourcing blood culture bottles, which was echoed by the lab professionals interviewed for this article.
“As of right now, the only thing we are on allocation for is blood culture bottles,” Panton commented. “We have some shortages in blood collection tubes and occasionally on some plastics. Overall, shortages in general are decreased.”
Blooflat too said her lab has been experiencing issues sourcing blood culture bottles, causing her team to “question doctors when they order blood cultures.” She explained:
“While the normal practice has always been to order two sets of blood cultures, we've been having to restrict our providers to just one set. They’ve been fighting it, of course, because they know this isn’t the best course of action. But what else can we do?”
Ferguson reports her lab has experienced shortages in blood culture tubes and PH testing reagents that are not commonly used by clinical labs. “We’ve also seen an increase in back orders and delays for a variety of supplies,” she added.
Odette Muriel-Ramos, Lab Manager, Keystone Urology Specialists, Lancaster, PA, said her lab’s supplier is currently switching them from the BD vacutainer gray topped urine culture tube “that has been around for probably 50 years,” to a “sponge that gets dipped in the urine.” Commenting on this change, she stated:
“I'm still waiting to hear from the supplier on whether they are changing the methodology given they have changed the medium. We need to know these types of things so we can evaluate collection options appropriately. The supplier is moving faster than what we are being educated.”
Ferguson also spoke to how the high cost of supplier shipping fees and poor equipment quality impacts her lab’s operations, stating:
“We will need to increase our lab testing fees due to higher cost of shipping charged by vendors and rising costs of equipment, including maintenance and repair. We estimate shipping costs have increased by 30% and more. In some cases, shipping costs are higher than that of reagents/supplies. In our experience, some refrigerators, freezers, incubators, and dishwashers recently manufactured by reputable companies have been inferior in quality. Equipment that should last for 10 years or more are replaced more frequently.”
Cardwell also emphasized the growing importance of supply chain considerations with the FDA’s increased oversight of LDTs. Laboratories will need to comply with new design controls, purchasing controls, and acceptance activities, which differ significantly from the current CLIA regulations.
“These new requirements are quite different from what exists under CLIA, and most laboratories lack experience in these FDA-required areas,” Cardwell said. “Lab professionals should familiarize themselves with these requirements and integrate them into practice.”
Additional opportunities and challenges with MDx testing
Those individuals interviewed for this article spoke to other trends and issues they are experiencing with MDx testing not covered in the survey questions. Here are some of their comments:
Laboratory technologist skillsets
Cardwell pointed to a shortage of molecular technologists with the skills needed for human genomic DNA testing, noting, “There is a real shortage of individuals skilled in next-generation sequencing, qPCR, and digital droplet PCR, where meticulous bench skills are crucial.”
Connectivity to maintain control
“As more solutions move into decentralized settings, connectivity solutions that can help customers maintain control and better manage the instruments and operators within these settings are critical,” said Patton. “In addition, connectivity solutions can enhance the efficient management of decentralized platforms by ensuring software remains up-to-date and automating the transmission of results.”
The role of physicians and other clinicians
According to Panton, while most labs are trying to add more molecular tests to their menus, because tests are costly, it is hard to justify these purchases with financial departments.
“Strong MD support (demand and orders) for the tests can help,” said Panton. “Administrators want to know how many FTEs will go away if we introduce these ‘rapid’ tests. With our current short staffing, it is not likely that these new tests will reduce any staff. In fact, they may need to add staff for performance and supervision. If we propose the MDX be installed as POC, then we need systems to train and manage a large nursing staff. Our MD support needs to show that we will have shorted LOS or lower drug usage with the advantages of MDx.”
Turning to running tests at the point of care (POC), she said this is “not always an advantage,” stating:
“It is hard for lab staff to manage the staff of other departments. RNs, aids and medical staff are not good at tracking tests, checking expiration dates, proper storage and even getting the results into the EMR and billing it.”
Muriel-Ramos has struggled to gain consensus among physicians that MDx testing is “the way to go,” given their reluctance to give up “old school cultures” and reimbursement concerns with new modalities.
“I’m fortunate that where I work now, the medical director loves genetics. He is one of the highest genetic ordering doctors in the United States. My goal is to bring PCR testing into the urology office but that requires securing approval from nine other doctors who had a poor experience when our office had attempted urine PCR in house testing in the past.”
“I found a company that does molecular for urine, I created an algorithm as to who gets sent out for culture versus who gets sent out for PCR, and a physician office policy where the lab can now decide which modality to use,” Muriel-Ramos added. “So, I feel like we're getting there, but we're not there yet because it is perceived among physicians as a big risk.”
Environmental impact
Blooflat voiced her concerns around the volume of waste generated by MDx testing, noting its impact on the environment. She stated:
“Single use kits and cartridges are a good thing because they provide a control for contamination, but we are throwing away a lot of plastic. I think someday it's going to have to be addressed. We're going to have to find a way to decontaminate these items and get them into the recycling system somehow.”
Looking ahead
MLO asked suppliers in the MDx space for their thoughts on testing trends in the year ahead and beyond.
“I see MDx testing becoming more personalized in the next year and into the future – if not to the individual then for groups of individuals,” said Kelly. “With better data from MDx, we can more rapidly gain knowledge about a specific cancer or a virus variant and more quickly determine the right treatment.”
“Looking ahead to 2025, laboratorians, clinicians, and leaders in healthcare settings can find value in molecular point-of-care testing,” said Patton. “With access to faster tests with high-quality PCR within decentralized settings, hospitals and health systems can truly rethink the patient care experience and support more efficient patient management and treatment.”
Patton said providers will also have more options for greater flexibility and customization of panel menus in the molecular lab, stating:
“This increased flexibility will also come with the potential for automatic or manual reflex options that will allow for multi-stage analysis and release of results for the necessary targets as defined for the individual patient. Improved flexibility has great promise to contribute to improved patient outcomes and diagnostic stewardship for the unique patient populations served by each institution.”
“At Revvity's EUROIMMUN, we are continuously advancing in step with the evolving field to deliver innovative and effective solutions that address emerging public health needs,” said Dr. Weiss. “As part of this commitment, we are adapting our molecular assays to meet new clinical challenges. For example, we have updated our assays to detect Trichophyton indotineae and other resistant dermatophyte species, as well as Candida auris resistant strains. These advancements ensure we stay at the forefront of diagnostic technology, providing crucial support in managing emerging infectious threats.”
References
1. FDA takes action aimed at helping to ensure the safety and effectiveness of laboratory developed tests. U.S. Food and Drug Administration. Published August 9, 2024. Accessed September 26, 2024. https://www.fda.gov/news-events/press-announcements/fda-takes-action-aimed-helping-ensure-safety-and-effectiveness-laboratory-developed-tests.
2. May L, Robbins EM, Canchola JA, Chugh K, Tran NK. A study to assess the impact of the cobas point-of-care RT-PCR assay (SARS-CoV-2 and Influenza A/B) on patient clinical management in the emergency department of the University of California at Davis Medical Center. J Clin Virol. 2023;68:105597. doi:10.1016/j.jcv.2023.105597.
3. Hansen GT, Moore J, Herding E, et al. Clinical decision making in the emergency department setting using rapid PCR: Results of the CLADE study group. J Clin Virol. 2018;102:42-49. doi:10.1016/j.jcv.2018.02.013.
4. Berry L, Lansbury L, Gale L, Carroll AM, Lim WS. Point of care testing of Influenza A/B and RSV in an adult respiratory assessment unit is associated with improvement in isolation practices and reduction in hospital length of stay. J Med Microbiol. 2020;69(5):697-704. doi:10.1099/jmm.0.001187.
5. Garvey MI, Wilkinson MAC, Bradley CW, et al. Impact of a PCR point of care test for influenza A/B on an acute medical unit in a large UK teaching hospital: results of an observational, pre and post intervention study. Antimicrob Resist Infect Control. 2019;8(1):120. doi:10.1186/s13756-019-0575-6.
6. Patel P, Laurich VM, Smith S, Sturm J. Point-of-care influenza testing in the pediatric emergency department. Pediatr Emerg Care. 2020;36(11):515-518. doi:10.1097/PEC.0000000000002250.
7. Youngs J, Marshall B, Farragher M, et al. Implementation of influenza point-of-care testing and patient cohorting during a high-incidence season: a retrospective analysis of impact on infection prevention and control and clinical outcomes. J Hosp Infect. 2019;101(3):276-284. doi:10.1016/j.jhin.2018.11.010.
8. Overview of FDA Laboratory Developed Test Final Rule. ASCP. Published May 14, 2024. Accessed September 26, 2024. https://www.ascp.org/news/news-details/2024/05/14/overview-of-fda-laboratory-developed-test-final-rule.
About the Author

Kara Nadeau
has 20+ years of experience as a healthcare/medical/technology writer, having served medical device and pharmaceutical manufacturers, healthcare facilities, software and service providers, non-profit organizations and industry associations.