The position of clinical laboratory director is challenging and comes with many responsibilities; the fundamental ones are maintaining quality standards and providing reliable test results. All lab directors face issues of delegation—what to delegate, and to whom? Part-time lab directors face particular challenges. In essence, they have all of the responsibilities of the full-time director—and they must meet them from an off-site position.
CLSI1, CLIA2, COLA3, CAP4 and other state-level regulatory bodies have specifically defined the roles and responsibilities of the clinical lab director. The director shall serve the laboratory either full-time or part-time. With regard to the latter, states have different regulations on the number of labs a part-time lab director can serve. Even if serving part-time, the lab director is responsible for the overall administration and operation of the laboratory. One of the major challenges part-time lab directors face is how to effectively monitor each aspect of lab operations while working remotely. As per CLIA, part-time lab directors can delegate selected duties to qualified personnel.2 Delegation doesn’t imply reduced accountability; it simply means transferring certain responsibilities to qualified lab personnel and giving them the authority that is required to fulfil those responsibilities.
General guidelines
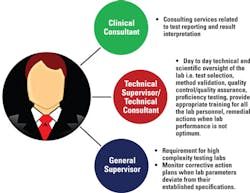
As outlined in Figure 1, part-time lab directors can delegate duties associated with results reporting and interpretation to the clinical consultant. Similarly, scientific and technical responsibilities of the lab can be delegated to the technical supervisor. In high-complexity labs, a general supervisor is appointed to monitor situations when a lab system deviates from its optimum parameters and to take appropriate corrective actions. However, the laboratory director is ultimately responsible for ensuring these responsibilities are properly performed.
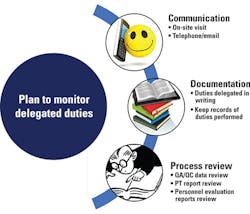
Different modes by which delegated duties can be monitored effectively are summarized in Figure 2. It is recommended that all the details of delegation be conveyed in writing, and the part-time lab director needs to make sure that the designees fully understand their roles and know to communicate with him or her if there is any doubt.2 Alternative candidates must also be assigned, in case of emergency and/or when the designated individual is not able to perform the delegated duties. The lab should maintain a delegation plan signed by the lab director and the person to whom the duties are delegated.1 This is an official record which brings clarity to the entire system and minimizes ambiguity among lab personnel regarding their share of duties.
Due to the structural and functional complexities of clinical laboratories, it is inherently challenging for the part-time laboratory director to monitor the entire lab workflow. For that reason, he or she should establish effective communication among lab personnel and other management staff. Communication can be made by direct on-site visits or by emails and telephone calls. Although CLIA doesn’t define how often a part-time lab director needs to visit the lab, a quarterly onsite visit is crucial for the part-time lab director to remain familiarized with lab operations and to monitor delegated duties. During each visit, he or she needs to review all laboratory documents and inspect the entire work process and work space. A report should be created after the visit which should include the number of hours spent in the lab, findings obtained from the review process, and possible corrective actions, if needed.
More broadly, documentation is another vital tool for part-time lab directors to demonstrate that they are running the lab effectively. There is truth in the saying, “If it was not documented, it never happened.” Everything—the delegation plans, the strategies for monitoring the plans, and the implementation of the monitoring strategies—should be very well documented.
Quality issues
As noted above, duties can be delegated, but responsibility cannot. It is recommended that part-time lab directors develop a plan to monitor the effectiveness of all delegated duties. Per CLIA, lab directors are required to review all quality control and quality assessment documents carefully on a routine basis to assure that all the parameters are optimal as defined by the lab and that physicians are receiving accurate and reliable results on a timely basis. Sometimes monitoring QA/QC documents alone is not enough to identify deep-rooted problems in the system. In such cases, a more stringent program needs to be established, and every process of the lab needs to be closely monitored.
For example, in the case of complaints against the lab being expressed by the client, the lab director needs to take appropriate action to resolve the underlying issue, and appropriate corrective actions need to be taken to minimize the chances of the problem occurring again.
Also, the director’s involvement is required for laboratory inspections and correspondence with government agencies and accreditation bodies. Although it is not mandatory for the director to be present in the lab at the time of inspection, it is his or her responsibility to create post-survey reports and/or corrective reports and submit them to the requesting agency.3,4
Another important fact is that not all duties can be delegated. Some responsibilities require the direct oversight of the director, full- or part-time. The director should ensure that sufficient and appropriately designed lab space is available for the testing performed and that the lab is complying with the Hazard Communications Standards. It is solely the director’s responsibility to make sure that a sufficient number of appropriately educated, experienced, and trained personnel are hired and that they perform their duties as defined by the lab. Also, in high-complexity labs, it is the director’s responsibility to ensure that a general supervisor is available for supervising other lab personnel. In addition, the director must ensure that the standard lab operating procedures are updated as needed and are followed by all personnel.
Most important, it is the lab director’s duty to ensure the quality of the entire test work flow.
It is evident that being a lab director is challenging, especially for part-time lab directors who are serving multiple labs. Not only do they have to perform lab director duties; they must also monitor that the delegated duties are performed efficiently. Proper communication and management are crucial to assure that the laboratory contributes effectively to clinical care and patient safety.
REFERENCES
- Quality Management System: Leadership and Management Roles and Responsibilities; Clinical and Laboratory Standards Institute, 2012.
- CLIA, 1988; Brochure 7.
- COLA Laboratory Accreditation Standard, May 2016.
- College of American Pathologists Accreditation Program Checklists, 2017.
Varsha Meghnani, PhD, serves as molecular geneticist and laboratory director for North Carolina-based Laboratory Start-Up Consultants (LSC).