In today’s world, with the ease of travel and individual mobility, healthcare data must travel with its owners (patients). However, nearly one third of patients are burdened with providing their own health record when seeking care. The goal of interoperability in healthcare is to make the right data available, to the right people, at the right time, across products and organizations in a way that can be relied upon and meaningfully used by recipients.
The other benefit of sharing data is creating venues for innovative research. Consumer electronics, with a variety of sensors, are tracking elements that affect our health, and more people are using apps to help manage their diet and exercise goals. Investigators are using this data to create solutions to several issues, but there are too many hurdles in making patient data portable, in a secure manner. The coronavirus disease 2019 (COVID-19) pandemic has exposed huge challenges in data sharing with which healthcare institutions had to comply. 1,2
Labs first
As laboratorians, we were the first to deal with information systems. Our instruments do not print data on a piece of paper anymore. Building interfaces and laboratory information systems (LIS) is part of our job. In fact, we are responsible for creating most of the data in a healthcare system. We also were the first to use and implement standards. Although we go to great lengths to make sure we correctly run our assays, we report them differently, even when using the same instruments. Not to mention that the reference range for any test with numerical results is different from one laboratory to another. We have very few assays with portable results between labs due to lack of availability of an international reference.
Focus on molecular pathology
The more complex a test is and the more data it generates, the more difficult the transmission of data to an information system becomes. Molecular testing is the cornerstone of therapeutic decision-making in the care for cancer patients, not only for choosing drugs but also to adjust drug dose. Clinicians rely on genetic tests results. Molecular assays are the most complex in our arsenal, considering the number of steps for data analysis and the different file types involved. Like most assays, we only provide an interpretation and do not share intermediary files in the electronic health record (EHR). Currently, there is no mechanism to share regions of the genes being tested and variants in an interrogable fashion. There should be a mechanism for reevaluating the results in context of new clinical and biologic information. None of these requirements can be accomplished with the static text-based report that we currently generate.
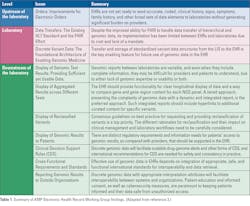
In late 2019, the Association for Molecular Pathology (AMP) Board of Directors called for the formation of an Electronic Health Record Interoperability for Clinical Genomics Working Group with expertise in molecular pathology and clinical informatics to recommend solutions about ways to resolve the current problems associated with the display and use of genomic data in EHRs. The working group performed a comprehensive examination of current problems and barriers to the display and use of genomic data in EHRs. Multiple problems were categorized and described, in detail, by the working group members. 3 The problems and opportunities were classified as EHR capabilities, upstream source and downstream recipient of data, and inherent issues of the LIS specifically related genomics and molecular pathology. Major themes were the lack of standards and consensus in current processes. (Table 1)
Role of FHIR
The article also reviewed, in detail, current initiatives and solutions. Noteworthy among these tools is FHIR (Fast Healthcare Interoperability Resources). It allows flexibility in the development of task-specific apps to interact with the EHR, which have4,5 become monolithic and the domain of only a few companies. FHIR adopts non-proprietary web technology called application programming interfaces (APIs)/web services. FHIR can be used as a stand-alone data exchange standard but can also be used with existing standards. However, HL7 FHIR and currently deployed HL7 version 2 are significantly different, and it will be costly for vendors and laboratories to switch to FHIR. In addition, there are no legal mandates requiring that communications between EHRs and laboratories use FHIR, unlike the older version 2 of HL7, which is required for use by certified EHR technology in the United States.
In summary, standards and tools are becoming available to create viable processes for interoperability and data sharing. However, reaching consensus and universal adoption of them takes time, resources, and capital.
References:
- The Office of the National Coordinator for Health IT (ONC). Interoperability. https://www.healthit.gov/topic/interoperability. Updated September 28, 2021. Accessed November 10, 2021.
- Healthcare Information and Management Systems Society (HIMSS). Interoperability in healthcare. https://www.himss.org/resources/interoperability-healthcare. Accessed November 10, 2021.
- Carter AB, Abruzzo LV, Hirschhorn JW, et al. Electronic health records and genomics: perspectives from the Association for Molecular Pathology Electronic Health Record (EHR) Interoperability for Clinical Genomics Data Working Group. J Mol Diagn. 2022 Jan;24(1):1-17. doi: 10.1016/j.jmoldx.2021.09.009.
- The Office of the National Coordinator for Health IT (ONC). Interoperability proving ground. https://www.healthit.gov/sites/default/files/sync_for_genes_report_november_2017.pdf. Published on November 28, 2017. Accessed November 10, 2021.
- Fast Healthcare Interoperability Resources (FHIR). Release 4. https://www.hl7.org/fhir. Published on November 1, 2019. Accessed November 10, 2021.