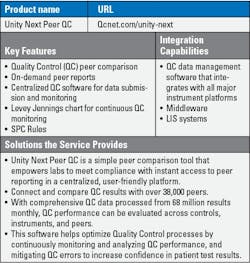
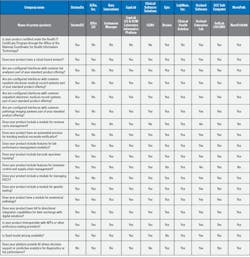
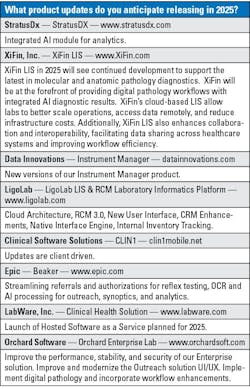
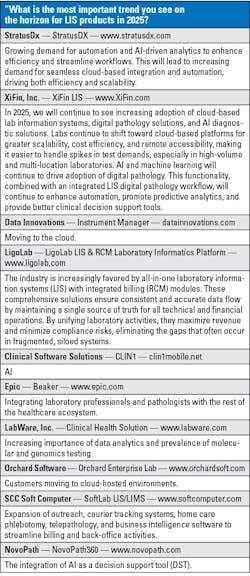
Choosing the right laboratory information system (LIS) is a challenging process for laboratories everywhere. Recent technology advancements like artificial intelligence (AI) and machine learning, cyberattacks, and an increasing demand for cloud-based solutions have all affected the LIS market.
Medical Laboratory Observer (MLO) interviewed five LIS experts to better understand the current trends and challenges they’ve observed in the LIS market this year.
Gilbert Hakim, CEO of SCC Soft Computer said, “We saw a significant increase in genetic testing performed in client labs instead of sending it to reference labs. Of course, the labs need to understand that the EMR vendor cannot correctly bill for these specialized tests and should rely on the LIS vendor's billing services to do so. We can actually do this billing very well. And we can now perform these billing services for customers with a different LIS.”
Suren Avunjian, LigoLab Co-Founder and CEO highlighted the rise of cyberattacks in 2024. “This spike in attacks underscored the urgent need for enhanced cybersecurity measures. As we head into 2025, implementing strategies like two-factor authentication (2FA), immutable backups, and comprehensive disaster recovery plans are no longer optional but essential components of a robust cybersecurity posture.”
“One of the trends that we saw in 2024 was the fact that labs are having to deal with staffing shortages and are turning to technology to help them do that—using the LIS to gain workflow efficiencies, adopting more automation,” said Kim Futrell, MLS(ASCP), MSHI, CPMM, Senior Strategic Marketing Manager, Orchard Software.
Other trends she noted were, “The adoption of digital pathology is causing labs to begin moving to anatomic pathology laboratory information systems with the ability to optimize digital pathology workflows,” and “The growth in molecular testing continued to drive the adoption of more comprehensive, mature, and reliable molecular information systems.”
Technology advancements
MLO asked LIS experts how recent technology advances have impacted LIS and what future developments they are excited about.
AI is one of the most significant advancements in the history of pathology, according to Joseph Nollar, AVP of LIS Product Management, XiFin, Inc. “During the pandemic, we saw an increase in requests to integrate digital pathology solutions. This was driven by the FDA changing its guidelines to make it easier to install systems so pathologists could work remotely. Post-pandemic, the primary driver of the growth in digital pathology has been FDA-approved diagnostic algorithms driven by AI. We are particularly excited about the continuing growth and expansion of diagnostic algorithms that will provide additional diagnostic insights, assist pathologists in their diagnosis, mitigate risk, and improve lab productivity. The LIS has a key role in facilitating a fully integrated digital pathology workflow with the ability to integrate AI-generated diagnostic results.”
Ed Krasovec, Director – Clinical Solutions, LabWare, Inc. said, “Because of the need to transform the large (and increasing) volumes of data that are being tracked into actionable information, data analytics generally is a hot area of interest across many categories of software, including LIS.”
Avunjian emphasized, “This is an exciting time as advancing technology supported by modern laboratory information system software has the power to push lab operations and finances to new levels of efficiency and improve patient care.”
He pointed to the following “positive impacts that AI and machine learning are already having on laboratory workflow management:
· Automated data entry: AI-powered OCR technology can extract data from handwritten or printed forms, reducing manual data entry, minimizing errors, and speeding up turnaround times.
· Automated data entry and coding: AI-powered systems can automatically extract relevant information from laboratory information systems and assign appropriate billing ICD and CPT codes. Machine learning algorithms can learn from historical data to improve coding accuracy over time.
· Workflow and process optimization: AI algorithms can analyze workflows to identify bottlenecks and suggest improvements, enhancing efficiency by automating repetitive tasks and integrating data from multiple sources.
· Automated result validation: Machine learning models can validate test results by comparing them against historical data, flagging anomalies, and reducing the likelihood of human error.
· Predictive analytics for denial management: Machine learning models can predict which claims are likely to be denied based on historical data. This allows lab billing teams to address potential issues before submission, increase claim acceptance rates, and reduce the time spent on resubmissions.”
Integration challenges
Navigating the change is one of the most common challenges laboratories face when integrating a new LIS, according to Nollar. “Lab staff are often resistant to change and may struggle with acceptance of a new system. Sometimes staff make requests to make the system behave like the old system that is being replaced, even when this undermines the new system’s operational improvements.”
He continued, “It is important to embrace your new system and all the new benefits and features that come with it. LIS providers, on the other hand, need to make sure that their system is adaptable to client needs regarding workflow and data configurations.”
Hakim and Krasovec both said resources are a barrier. “Common challenges include difficulty in devoting necessary resource bandwidth of key subject matter experts to the implementation to provide input to the project, underestimating the effort related to inputting the lab’s master data dictionaries, and the tendency to replicate a legacy LIS solution rather than embracing the out of box behavior of the new LIS solution,” Krasovec said.
Hakim added, “These systems are being replaced because they are antiquated, and the vendor is not advancing the system to accommodate today's demands on an LIS. With laboratory staffing shortages, we focus on automation to reduce the amount of human intervention required to perform testing workflows. Often, we augment customer staffing to implement the new laboratory and genetics software to meet timelines. To expedite implementation, we use a best-practice model database for dictionaries, instruments, and workflows to reduce the timeline and cost.”
Data security
Futrell pointed to the significance of data security to healthcare information systems and shared best practice strategies Orchard Software recommends for data management. “Orchard Software recommends that our solutions be deployed in a healthcare-centric data center that provides the secure design, business continuity, and disaster recovery that is required by federal healthcare data regulations.”
She continued, “If the solution is hosted by Orchard Software, our cloud-deployed solutions are housed in Amazon Web Services (AWS) cloud instances. AWS supports 143 security standards and compliance certifications, including PCI-DSS, HIPAA/HITECH, FedRAMP, GDPR, FIPS 140-2, and NIST 800-171, helping customers satisfy local, state, and federal requirements for data protection and security. Orchard Software maintains a SOCII type 2 certification status and leverages AWS certifications and accreditations, demonstrating compliance with rigorous international standards.”
Recent high profile ransomware attacks have highlighted the need for stronger cybersecurity measures because healthcare organizations, including clinical labs, are attractive targets for cybercriminals, said Avunjian. “To combat these threats, providers must first adopt their own robust cybersecurity strategies, and then, just as importantly, partner with laboratory information system companies that emphasize the importance of cybersecurity and offer practical and relatively inexpensive solutions.”
Cloud-based solutions and the future of LIS
When asked what role cloud-based solutions will play in the future of LIS, Krasovec said that “many labs are finding advantages in outsourcing their IT infrastructure to cloud providers so that they can focus on their lab operations and not on IT.”
“The benefits of doing this are significant: improved security and compliance, reduced IT manpower, improved scalability & flexibility, and more,” he continued.
On the contrary, “The challenges we see include confusion related to differences between cloud hosting, SaaS (Software as a Service), IaaS (Infrastructure as a Service), and PaaS (Platform as a Service) and what the implications are for each of these options to the lab with regard to their IT responsibility and the flexibility or lack thereof to customize their LIS application.”
Hakim said, “Over the past four years, requests for cloud-based LIS solutions have significantly increased. Some of our largest commercial labs have now deployed our LIS and Genetics software in the cloud. Many clients are shifting from “On-Prem” to cloud-hosted solutions. The most crucial factor is that the same software currently running in your LIS can be ported to a cloud-hosted solution. It is important not to introduce disrupters during this process. Most of our customers using courier, logistics, CRM, and supply chain management software are now cloud-hosted.”
Cloud-based solutions can increase the security of protected health information, Futrell added. “Offloading the bulk of security measures to a cloud vendor makes data more secure and reduces the burden on the organization’s IT staff. Healthcare organizations (HCO) that use a cloud-based information system can obtain a cost-effective IT solution without the capital layout or expenditure for internal IT staff to maintain and service the infrastructure. A subscription service levels the cost curve and eliminates costs peaks that occur across time. Cloud systems increase data redundancy and system availability (or uptime) by automating backups and disaster recovery options. This added data protection means that an HCO does not lose vital patient data and can minimize downtime.”
Considerations
Nollar concluded, “An LIS is one piece of the laboratory infrastructure puzzle. Often, it is selected, implemented, and used by those who have a primarily clinical lens. Sometimes, this approach omits the possibility of really advancing the lab’s automation from the first patient encounter to the lab result to a claim that is fully paid.”
“Today's LIS must consider all of the inputs needed by the leading revenue cycle management solutions to ensure that claims are submitted without errors and have the necessary supporting information for the claim to be adjudicated in a timely and efficient way. Today's LIS should be capable of transmitting the necessary information for claim submission, including prior authorization numbers from payors as just one example. Gone are the days of selecting, contracting, implementing, and using an LIS without considering the end-to-end experience for the ordering physician, the digitally savvy patient, and the downstream reimbursement and payment mechanisms.”