COVID-19 highlights need for laboratory data sharing and interoperability
Secure sharing of patient data across healthcare systems and organizations – better known as interoperability – is widely recognized as part of the solution to improve patient outcomes. The laboratory is an important part of the move to interoperable healthcare systems because of the tremendous amount of data it manages and the value of that data in making patient care decisions. The SARS-CoV-2 pandemic has made the need for an interoperable healthcare system blatantly evident.
COVID-19 reiterates the value of interoperability
Transmitting data on COVID-19 results to government officials often remains a manual process involving paper and faxing. Currently, each state has its own guidelines for COVID-19 response, so statistics are inconsistent and vary based on the information source and location.
An interoperable healthcare system would greatly improve the nation’s response to COVID-19. For instance, a patient with symptoms could visit his or her provider, and the provider would have immediate access to their full medical record to address any underlying conditions. This scenario would alleviate time wasted tracking down records and reduce or eliminate duplicate testing. When a patient with COVID-19 moves from primary care to emergency care, quick decisions and tracking of laboratory results is necessary. Shared data improves the providers’ ability to provide prompt and effective patient care.
Interoperability requires removal of data silos
The ability to deliver targeted clinical interventions efficiently and effectively is a key aspect of healthcare value. To facilitate this value, providers need access to comprehensive, timely patient data, including information about existing chronic conditions that may affect treatment decisions. A significant factor in the lack of shared patient data is the healthcare industry’s data silos that can make patient care cumbersome and, in some cases, dangerous.
Interoperability allows the sharing of data from electronic health records (EHRs) and other information systems across organizations and geographic locations. If patients travel to another state for vacation, then go to the emergency department (ED), their records from their home healthcare provider (from another state) are readily available in the ED, as those systems are electronically connected and able to share data. With this need evident, interoperability has been long-awaited in healthcare, and there is finally some effort in this direction.
Eliminating Interoperability barriers is slow
Yet, progress toward a connected healthcare system is slow. The U.S. healthcare system has spent billions of dollars to incorporate the use of EHRs through the Meaningful Use program, now renamed Promoting Interoperability, making their use commonplace in hospitals and within the majority of ambulatory provider offices.
Laboratory information systems predate EHRs and have been a strong component of laboratory workflow for decades. Often within healthcare organizations, these two information systems are interfaced and share data, creating a foundation for data sharing. However, the industry has made less progress in connecting systems and data among providers and across geographic locations. The technology is available, but data is still is not easily shared.
The Promoting Interoperability program relies on provider grants and incentives and does not encourage data sharing. In addition, the U.S. healthcare reimbursement system remains fragmented and does not promote interoperability. Only where revenue comes into play does integration occur. For example, most medical claims are transmitted electronically, because this accelerates payments. Laboratories are generally well-connected to the internal providers who order tests, because this link supports reimbursement.
ELR: Key to pandemic response and core to healthcare interoperability
Electronic Laboratory Reporting (ELR), the electronic transmission of laboratory data from laboratories to public health entities for reportable conditions, was included as part of the Meaningful Use program and has been in place for quite some time in many healthcare organizations.
The Laboratory Response Network (LRN), created by the Centers for Disease Control and Prevention (CDC) more than two decades ago, uses a variety of networks to handle emerging threats, such as a pandemic. The primary function of the LRN is to provide rapid detection of bio-threat and emerging agents of infectious diseases. The strength of the LRN lies in its standardized approach and its tiered capability construct – with sentinel clinical laboratories serving at the foundation to quickly recognize, rule-out, or refer potential bio-threat agents to the LRN reference laboratories. The LRN structure for biological threats is national laboratories, reference laboratories, and sentinel laboratories, including more than 150 state and local public health, military, international, veterinary, agriculture, food, and water testing laboratories. LRN labs must report their results to the CDC through an electronic portal called the Public Health Information Network Messaging System (PHIN-MS).
The LRN receives about 20 million reports annually, of which approximately 80 percent are electronic, via an HL7 interface. Nevertheless, participation in electronic reporting remains uneven, with larger labs reporting electronically, but hospital labs less likely to do so.
21st Century Cures Act pushes interoperability but faces delays
The 21st Century Cures Act, signed into law in December 2016, was also a push toward nationwide clinical data sharing. The Cures Act intends to provide $6.3 billion over a decade to the National Institutes of Health (NIH) for the Precision Medicine Initiative, Cancer Moonshot, BRAIN Initiative, and regenerative medicine using adult stem cells.1
Part of the act’s overarching goals are to achieve widespread interoperability among health IT systems and improve patient accessibility to their medical information. The Cures Act includes specific measures that promote these initiatives, and they’ve been implemented through separate rules issued by the Office of the National Coordinator for Health Information Technology (ONC) and the Centers for Medicare & Medicaid Services (CMS).
Due to the COVID-19 pandemic, in October 2020, ONC announced a second extension of the timeline for implementation of its regulations related to the 21st Century Cures Act until 2021.2 Enforcement for specific regulations implemented by CMS ranges from 2021 to 2022.3
Laboratory interoperability requires standards
A key element of laboratory interoperability is the use of terminology standards that reduce the time spent exchanging, tracking, and reporting tests. Observational data findings from a provider encounter, laboratory data, and other diagnostic information are translated via standard terminologies that can be understood by all information systems. These terminology standards include LOINC and SNOMED-CT codes.
Additionally, there is a collaborative group called the Systemic Harmonization and Interoperability Enhancement for Lab Data Shield (SHIELD) that is working to “improve the quality, utility and portability of electronic laboratory data through the harmonized implementation of semantic data standards that have been appropriately qualified by a sole authoritative source.”4 SHIELD, which is an initiative of the Medical Device Innovation Consortium, supports the provision of standardized codes from manufacturers to laboratories to consistently identify tests in the LIS and EHR. This standardization allows laboratory data to be consistently interpreted by information systems, thereby promoting interoperability. Improving the semantic interoperability of laboratory data between organizations allows diagnostic information to be more efficiently used to support clinical decisions.
There is also a COVID-19 Interoperability Alliance, which is a collaboration between healthcare industry stakeholders to provide a collection of value sets for clinical, demographic, and administrative terms relating to COVID-19. The Alliance’s goals are to provide these resources to the healthcare industry to support data aggregation and interoperability that will allow the nation to gain a greater understanding of the pandemic.5
The CARES Act addresses lab data reporting
Laboratory testing is one of the most significant parameters necessary to track and mitigate a pandemic. Public health management of the COVID-19 response needs to be highly organized, including accurate laboratory testing, standardized test results, data sharing, and the capture of demographic information to allow for contact tracing, mitigation, and control of the virus.
In response to the SARS-CoV-2 pandemic, the Coronavirus Aid, Relief, and Economic Security Act (CARES Act), a $2.2 trillion economic stimulus bill, was signed into law in March 2020. The CARES Act intends to provide timely economic assistance to American families and small businesses, addressing a number of areas of need (e.g., direct payments, unemployment, payroll taxes, retirement funds, support for hospitals, PPE, testing supplies, vaccination costs, etc.).
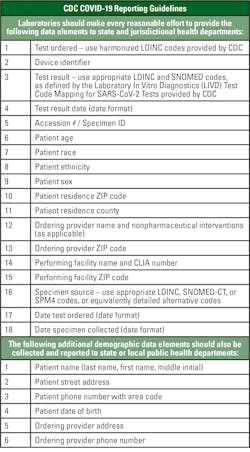
A portion of the CARES Act, Section 18115, requires laboratories to report results of COVID-19 testing daily to the Secretary of the Department of Health and Human Services and appropriate state or local public health departments. See Figure 1 for the required data elements.6
Data sharing needed to advance healthcare system and address pandemic
Lack of data interoperability in healthcare, with clinical and financial data residing across different systems, has caused extensive challenges for many years. Disparate data has prevented both payers and providers from achieving a comprehensive view of the patient record and slowed the shift to value-based care. Lack of shared data has also diminished the healthcare industry’s ability to proactively respond to the COVID-19 pandemic.7
Quickly and effectively communicating lab orders and results across care locations and between treating providers is of extreme value in patient care. Sharing diagnostic information through integration of the LIS to other information systems, including the EHR, is a crucial cornerstone of interoperability. Sharing patient data is also a foundational need for the management of public health crises, such as COVID-19. The importance of interoperability needs to be understood, communicated, and embraced within the healthcare system, backed with regulatory measures and an incentive structure that encourages interoperability between vendors and healthcare organizations.
References
- Green J. What the 21st Century Cures Act means for the EHR market. EHR in Practice. December 2016. www.ehrinpractice.com/what-the-21st-century-cures-act-means-for-the-ehr-market.html. Accessed January 28, 2021.
- Information blocking and the ONC health IT certification program: extension of compliance dates and timeframes in response to the COVID-19 public health emergency. Federal Register. November 2020. www.federalregister.gov/documents/2020/11/04/2020-24376/information-blocking-and-the-onc-health-it-certification-program-extension-of-compliance-dates-and. Accessed January 29, 2021.
- Reducing provider and patient burden by improving prior authorization processes and promoting patients’ electronic access to health Information. Centers for Medicare & Medicaid Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Interoperability/index. Accessed January 31, 2021.
- Systemic harmonization and interoperability enhancement for lab data (SHIELD). June 2020. Medical device innovation consortium. https://mdic.org/program/systemic-harmonization-and-interoperability-enhancement-for-lab-data-shield/. Accessed January 28, 2021.
- COVID-19 Interoperability Alliance. https://covid19ia.org/. January 29, 2021.
- How to report COVID-19 laboratory data. Centers for Disease Control and Prevention. January 2021. www.cdc.gov/coronavirus/2019-ncov/lab/reporting-lab-data.html. Accessed January 29, 2021.
- Rizk E. Three ways analytics-fueled interoperability is crucial during COVID-19 and beyond. AHIMA. October 2020. https://journal.ahima.org/three-ways-analytics-fueled-interoperability-is-crucial-during-covid-19-and-beyond/. Accessed January 29, 2021.