U.S. medical labs embrace digital transformation amid staffing, cost and data challenges: Key insights from the 2025 MLO State of the Industry (SOI) Survey
U.S. medical laboratory professionals continue on their digital transformation journey, moving from on-premise, legacy laboratory information systems (LIS) to hybrid and cloud-based solutions, digitizing more processes, and increasing use of analytics to support operations, according to the results of the 2025 MLO State of the Industry (SOI) Survey Article on Data Analytics.
On the other hand, their advancement remains hindered by factors including disparate systems, data silos, staffing challenges, inadequate information technology (IT) support, and cost constraints.
Five key findings:
1. Costs and staffing challenges top of mind: 71% ranked staffing and 66% funding high on their lists of challenges in short-term planning and forecasting. 64% report measuring cost per test as an operational performance indicator in their labs (up from 54% in 2024), and 47% report measuring staff productivity goals (up from 39% in 2024).
2. Greater digital functionality: 12% more respondents report their labs’ regulatory compliance/reporting is electronic through their LIS (34% in 2025, up from 22% in 2024), and 11% more have electronic point of care testing (POCT) management functionality (41% in 2025, up from 30% in 2024), compared with last year.
3. Desire for more data analytics: There was an 18% increase in respondents reporting they are utilizing data analytics to support some aspects of lab operation and management and are planning more (54% in 2025, up from 36% in 2024).
4. Shift away from on-prem LIS: 18% fewer survey respondents reported using in-house software/servers compared with last year (52% in 2025, down from 70% in 2024).
5. System and data challenges remain: Although slightly fewer respondents reported interoperability and data integration issues with their LIS or electronic health records (EHR) systems are holding them back from digitizing processes, close to half of lab professionals (48%) still struggle with these stumbling blocks.
The survey averaged 106 respondents with most laboratory professionals working in hospital laboratories (59%) and holding lab manager, administrator or supervisor positions (32%). Nearly one-quarter (24%) report working in labs with 1-10 employees, nearly one-third (28%) in labs with more than 100 employees, and the remaining 48% in labs with staff sizes in between.
Regarding annual volume of testing performed in their labs, 9% report less than 25,000 tests on the low volume end of the spectrum and 23% report more than 2M tests on the high end. More than one-third (31%) process between 500,001 – 2M tests per year, and the remaining fall in between 25,001 and 500,000 annually.
Priorities and planning
When asked to select their organization’s top strategic IT priority in the next three years, infrastructure and platform development and new LIS garnered the highest responses, at 19% and 18% respectively.
Data analytics optimization to support lab management was next on the list of priorities (15%), followed by revenue cycle management optimization (11%), digital pathology implementation (10%), integration of the EHR (9%), and POCT product (3%).
Additionally, 16% of lab professionals selected “other” when answering this question, commenting on top strategic IT priorities not offered in the survey. This included, new reference lab interfaces, more auto verification from analyzers, interconnectivity with reference and public health labs, and a bi-directional system to integrate with a health system’s hospitals.
“We see laboratory organizations and diagnostic providers implementing new, more scalable platforms and consolidating existing ones as a top strategic priority,” said John E. Johnson III, Senior Vice President, Sales, ELLKAY. “Laboratories are back operating in a world where their test menus are expanding and managing the entire test menu is important.”
Johnson has seen more “focused priority and activity” around lab technology infrastructure improvements, specifically in large reference laboratories, health system laboratories and specialty diagnostics. He stated:
“LIS and the interoperability platforms that feed data to them are IT priorities for organizations that are expanding their laboratory services. Health system laboratories and community hospitals with lab services are consolidating from multiple LIS to single platforms across all specialties.”
According to Johnson, these IT infrastructure improvements are multi-year initiatives that take careful strategic planning for IT and business teams. “Labs are uplifting platforms, replacing technology, and modifying processes that have been in place for many years, all while continuing to serve their providers and patients.” he explained. “Laboratories are looking for an experienced IT partner that offers a combination of technology advantage and laboratory experts that can advise and do the work with less disruption to the business and the laboratory professionals that are focused on delivering services and care.”
Forecasting
With regards to management forecasting, 68% of survey respondents reported their labs perform test utilization prediction, 55% staffing levels, 55% workloads, 27% supply utilization, 20% increased case volumes, and 9% other.
Comparing this year’s results to last year’s, there was a 9% jump in respondents reporting management forecasting of staffing levels (55% in 2025, up from 46% in 2024), and a 9% drop in those reporting forecasting of supply utilization (27% in 2025, down from 36% in 2024).
There was a slight increase in lab professionals reporting use of an electronic tool for management forecasting, 27% in 2025, up from 24% in 2024.
Challenges
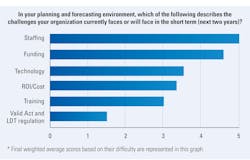
Regarding challenges their organization currently faces or will face in the short-term (next three years) in their planning and forecasting environment, staffing topped the list, with 71% of lab professionals ranking it first or second. Funding was next, with 66% ranking it first or second.
Over one-third (32%) of lab professionals surveyed ranked return on investment/costs first or second in terms of challenges, while 17% ranked technology and 10% ranked training.
Only 5% ranked the U.S. Food and Drug Administration’s (FDA) Valid Act and Laboratory Developed Tests (LDT) regulation as their first or second greatest short-term challenge in their planning and forecasting environment.
“The biggest challenge is that some clinicians are not that interested in the running of the lab, just the results,” said Fred Morley, MA, MT(AMT), Certified Technical Consultant (ACHC), Fred Morley Services. “I wish there would be more strict regulations about yearly continuing education for their responsibilities in the lab. I would like to see COLA establish a rigid certification program for the technical consultants (TC) with examinations, unlike American Medical Technologists (AMT).”
Systems, processes and data
Surveying technology systems and data, over half of respondents (52%) report using software/servers in-house for their LIS infrastructure, which is an 18% decrease compared with 2024 (70%). There was also a drop in respondents reporting use of cloud-based LIS (20% in 2025, down from 28% in 2024).
New for this year, MLO offered hybrid LIS solution as an answer option for this question, with nearly one-third of respondents (28%) reporting use of this type of platform.
Morley commented on the LIS capabilities for the labs he consults for:
“As the technical consultant for six labs, only four have an LIS system. The other two just scan their lab results into the patients’ charts. Those with LIS mostly use it for patient results, not administrative analysis. I am slowly urging them into analysis as I know how it can be a basis for improvement. They would be most helped by cost per test and productivity.”
Electronic functions
When asked what functions are electronic through their LIS, 93% selected electronic orders and results, 82% integration with analyzers, 64% billing/revenue cycle management, 54% QA/QC, 41% POCT management, 34% regulatory compliance/reporting, 25% scheduling, 23% inventory control/supply chain management, and 20% customer service.
Comparing this year’s survey results to last year’s to identify any interesting shifts, there was a 12% increase in respondents reporting their regulatory compliance/reporting functions are electronic through their LIS (34% in 2025, up from 22% in 2024), an 11% increase in those with electronic POCT management (41% in 2025, up from 30% in 2024), and an 8% increase in electronic inventory control/supply chain management (23% in 2025, up from 15% in 2024).
Challenges
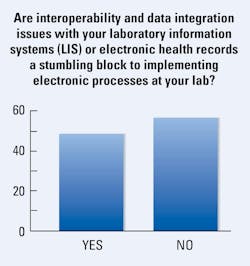
When it comes to challenges with interoperability and data integration between their LIS or EHR systems, nearly half (48%) of medical lab professionals reported these issues as a stumbling block to implementing electronic processes at their labs. This was a 5% drop from last year when 53% reported these problems.
MLO asked the survey respondents that are having interoperability and data integration issues to comment on the specific challenges they are facing. The comments fell into several categories:
· System deficiencies: Many respondents to this year’s survey wrote in comments related to systems lacking the capabilities they need, most notably, the inability to integrate. Comments included:
o “The issue is with the hospital system, not our LIS.”
o “[Vendor name] is our current EHR and they are very limiting on what they will work with us to implement.”
o “Time to implement new interfaces is lengthy.”
o “The medical records and LIS do not interface both ways.”
o “Interconnectivity issues between reference and public health labs.”
o “The current [EHR] does not have a blood bank module that will help integrate all results into one LIS.”
o “[Our] POCT software is old, trying to upgrade to better integrate with EMR is a challenge.”
o “Unable to connect to proficiency testing and some lab and POCT analyzers due to age of LIS.”
o “Running multiple middleware systems is a headache.”
· Lack of IT support: Lab professionals expressed frustration with insufficient IT resources in their organizations. Comments included:
o “IT decides which projects to work on.”
o “IT is backlogged with requests to update/change as the lab does.”
o “IT does not care about the laboratory one bit.”
· Cost restraints: Survey respondents voiced the challenge of cost when approaching solutions for lack of system interoperability and data integration. Comments included:
o “Cost is prohibitive to interface 500 clinic locations in a four-state region.”
o “[Cost of] vendor pricing to integrate and interface to outside systems.”
o [Our system] needs middleware we can't afford and there are other analyzers that just won't stay connected.”
o “[Integrating] LIS and electronic health records is very expensive for an academic institution.”
· Data and reporting issues: It was clear from the comments that many lab teams still struggle with manual data entry and manipulation for reporting. Comments included:
o “Must enter every data point or process manually.”
o “Even with an LIS, data mining is difficult, and [we] still need to export to Excel to manipulate data, very time consuming and not necessarily real time. Always after the fact.”
o “Limited functionality requires multiple manual process to extract and recompile data in order to manipulate into useful information.”
o “Information is too compartmentalized.”
o “Reports are difficult to generate as some are manual processes and some are electronic.”
o “Lack of interface and reporting capabilities.”
“One of the main challenges we face is integrating data from multiple sources into a cohesive and actionable format,” said Amanda J. Lewis MLS(ASCP)cm, Quality Assurance Supervisor Abilene Market Laboratories. “Different systems often use varied data formats and standards, making data integration and interoperability a complex task.”
“Additionally, ensuring data accuracy and consistency across systems is critical,” Lewis continued. “We also encounter challenges related to data privacy and security, as we must comply with stringent regulations while making the data accessible for analytics.”
“I’m not surprised that interoperability and data integration between LIS and EHR are top strategic priorities for survey respondents,” said Johnson. “This coincides with the trend in new LIS and data platform development to support analytics. Laboratories require access to more electronic data, and this is an opportunistic time to improve data integration between LIS and EHR.”
“There is a very active movement to improve data integration to drive automation and electronic process aimed at efficiently managing higher test volumes,” Johnson added. “Labs can’t optimize processes unless they optimize interoperability. The continued uptick in genetic and complex testing requires a more robust electronic dataset and this in turn requires that the interoperability of LIS and EHR improve.”
MLO also asked survey respondents who are not having interoperability and data integration issues to comment on success factors. Comments included:
· “The updates occur as needed.”
· “[We have a] separate lab module that is user friendly.”
· “Vendor is well known to our automated equip vendors.”
· “We've had electronic processes since 2015 and continue to expand them.”
· “[Our EHR) is used between multiple facilities.”
· “It takes time to build a new test or instrument, but the process is a work in progress. Satisfied with result.”
· “We have competent hands to support the system and process integration.”
Data analytics and operational KPIs
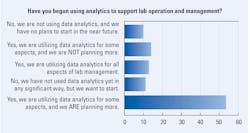
When asked if they had begun using analytics to support lab operation and management, lab professionals utilizing data analytics for all aspects of lab management dropped by 5% compared with last year (12% in 2025, down from 17% in 2024).
Although those who said they are utilizing data analytics for some aspects and are planning more shot up 18%, with more than half of this year’s respondents (54%) selecting this as their answer compared with 36% in 2024.
For those considering a move in this direction, 11% reported not yet using data analytics in any significant way but wanted to start, although this was down from 22% in 2024.
“Our lab is increasingly accessing data from our laboratory information system (LIS) and other integrated systems for analytics and decision-making,” said Lewis. “This access allows us to track and analyze various performance metrics, identify trends, and make data-driven decisions that enhance our operational efficiency and patient care outcomes.”
Among those with no interest in pursuing analytics or expanding what they have in place, 14% report they are utilizing data analytics for some aspects but not planning more (up from 10% last year), and 10% report how they are not using data analytics and have no plans to start in the near future (down from 12% in 2024).
“We are not increasingly accessing data from our LIS,” said Sherrie White, MBA, MHA, MT (ASCP), laboratory consultant. “We are at a plateau of what we access for quality metrics and volume reporting. The challenge is the lack of cost data that is within the LIS for laboratory testing. It would be great if we could enter a dollar amount associated with each test to see what actual spending is.”
Specifically for lab professionals who are using data analytics in their operations, MLO asked how often the data is refreshed. The top selection was monthly at 34%, followed by real-time at 26%, daily at 22%, weekly at 9%, minutes at 5%, and hours at 4%.
With regards to data analytics tools, 40% of respondents said they use a tool integrated with their LIS, 32% one that is part of their LIS, and 27% a separate tool.
Kevin Haas, chief technology officer (CTO), Myriad Genetics, commented on the ongoing evolution of digital transformation in the medical laboratory environment, stating:
“While we used to think of medical lab professionals wearing white lab coats and using pipettes, their job is turning into data generation and research. For many, their primary aim has become data analytics - taking information out of their LIS, combining it with the clinical indications for testing, combining that with the outcomes, and bringing in the omics data from sequencing and other data sources. “Therefore, what was in the past a biology problem has become a data problem, because you must be able to find the data, and it must be accessible, interoperable and reusable.”
According to Haas, all this data points to the challenge of the different platforms and systems that house it.
“It is a software engineering problem, he stated. “I think our industry is still in its adolescent phase, but other industries have overcome similar challenges. Back in the 1980s, computer companies had all these interoperability challenges, which prompted them to create open standards. The medical lab industry is now starting to do this. To figure out – ‘what is the data standard that we need to run a lab?’ Until then, every LIMs project is like starting from scratch, having to perform countless custom integrations to get things up and running.”
Haas said Myriad began tackling this challenge a decade ago in its own labs, building out a fully integrated environment to where today processes are highly automated from beginning to end.
“We have hundreds of different instruments that work in concert from a tube of blood, to advanced chemistry, to loading onto a sequencer, to fully automated data analysis,” he explained. “It is not until the later stages when humans specifically come into the loop to perform data interpretation and quality control. It really is the full vertical system integration that stitches together all the little pieces of information captured along the way.”
“When most people think of lab automation, they envision taking one step somewhere that is performed by human hand and programming a robot to do that step,” Haas continued. “Our fully automated approach involves designing for robotic processes first and leveraging human elements in places where complex abstract reasoning gives a justifiable reason to do so because that is what is necessary to run at scale and to accelerate scientific innovation.”
KPIs
Turnaround time topped the list of operational key performance indicators (KPI) the respondent’s labs measure, with 89% of lab professionals selecting this KPI. Next was quality improvement initiatives with 73% respondents selecting this metric.
Comparing this year’s to last year’s results, there were noticeable increased in survey respondents reporting measurement of cost per test (64% in 2025, up from 54% in 2024) and staff productivity goals (46% in 2025, up from 39% in 2024). Billable tests versus performed tests as a reported KPI increased as well (46% in 2025, up from 40% in 2024).
“Currently I see labs using data and analytics to optimize electronic processes and increase automation to improve efficiency,” said Johnson. “Automation inside the lab is a priority that is getting more attention. Automation is not a single year project but rather an initiative that evolves to align with a lab’s advancing infrastructure. I am encouraged at the progress I see, because it is about modernizing the lab, and the data infrastructure to support the advancements in diagnostics that are the foundation for the future of precision medicine.”
Those reporting measurement of unnecessary tests was down from last year (16% in 2025, down from 23% in 2024), as well as medical necessity (30% in 2025, down from 37% in 2024).
Commenting on her lab’s KPIs, Lewis stated, “We seek data on several key performance indicators, including cost per test, turnaround time, and staff productivity.” She described how this data helps them in several ways:
· Cost per test: “By analyzing the cost per test, we can identify areas where we can reduce expenses without compromising the quality of our services. This includes optimizing reagent usage, reducing waste, and negotiating better pricing with suppliers.”
· Turnaround time: “Monitoring turnaround times allows us to identify bottlenecks in our workflow and implement process improvements to ensure timely delivery of results. This is crucial for maintaining high levels of patient satisfaction and clinical efficiency.”
· Staff productivity: “Analyzing staff productivity helps us allocate resources more effectively, ensure balanced workloads, and identify training needs. It also allows us to recognize high-performing staff and address areas where additional support may be needed.”
· Error monitoring: “By comparing edited results and final anatomic pathology diagnosis, we are seeing where we can improve processes, training, and testing availability.”
“We would love to be able to have our LIS capable of calculating cost per test,” White commented. “This is the one area I feel could be added to LIS platforms.”
Lastly, looking at the types and number of tests tracked by medical labs, COVID-19 came out on top, with 73% of lab professionals reporting they track the number of COVID-19 tests performed (a slight change from 72% in 2024), followed by Influenza at 67% of respondents (up from 63% in 2024).
Compared with last year, there was an increase in lab professionals reporting they track the number of sexually transmitted infection STI/HIV tests performed, 45% in 2025, up from 40% in 2024, and a decrease in tracking the number of RSV tests, 49% in 2025, down from 59% in 2024. The number of strep tests tracked remained relatively the same year over year, 45% in 2025, down from 46% in 2024).
New for this year, MLO asked if those surveyed tracked the number of tests for healthcare associated infections (HAIs), with 32% reporting they do track this infectious disease category.
Looking ahead
Medical lab professionals and others interviewed for this article commented on how use of data and analytics is trending in 2025.
John E. Johnson III, Senior Vice President, Sales, Ellkay
"In the past 25 years, I've seen a convergence between laboratory diagnostics and precision medicine. This convergence is beneficial, driving the utilization of precision medicine. As a result, there is an increasing need for digital and data strategies. Diagnostics is proving its value due to the growing demand for data utilization in precision medicine diagnostics. More and more, labs are playing a bigger role in delivering precision medicine. The use of precision diagnostics is increasing to inform more targeted treatments, particularly for better patient care in oncology (e.g., lung and breast cancer) and chronic diseases like diabetes and obesity.”
“This has led to a significant focus on data in digital pathology. Early adopters are using data to improve efficiency and access for patients, providers, and experts by automating manual processes.”
Amanda J. Lewis MLS(ASCP)cm, Quality Assurance Supervisor Abilene Market Laboratories
“We see significant opportunities in leveraging AI and machine learning to predict trends, enhance diagnostic accuracy, and improve patient outcomes.” For instance:
- Predictive analytics: “AI can help us predict patient trends, such as the likelihood of disease outbreaks or the need for specific tests, enabling us to prepare and allocate resources more effectively.”
- Enhanced diagnostics: “Machine learning algorithms can assist in interpreting complex data sets, leading to more accurate and faster diagnoses. This can be particularly beneficial in fields like pathology and genetics.”
- Operational efficiency: “AI-driven automation can streamline administrative tasks, reduce manual errors, and optimize our lab operations. This includes automating routine processes like sample sorting and result reporting, allowing our staff to focus on more complex tasks.”
- Test algorithms: “By using standardized testing algorithms enhanced with ML and AI, better test utilization and less wasted testing could streamline processes, costs, and patient satisfaction.”
Sherrie White, laboratory consultant
“The integration of test cost information to the LIS would be very helpful. Pricing for the LIS systems themselves is going to be key in the future. With costs increasing and reimbursement decreasing, spending additional dollars on analytics gets tough. Having these analytics provided at no charge or included with the platform you use will be key to labs using them.”
Kevin Haas, chief technology officer (CTO), Myriad Genetics
Haas spoke to how many of the automation and data analytics techniques used in molecular diagnostics are leveraged in partnership with biopharma as well, including target identification, mechanism discovery, and preparing cohorts to test efficacy of the candidates with real-world data. He stated:
“At the meta level, the only thing that advances the speed of scientific discovery is increasing the iteration time by pulling in more data and performing more experiments faster. Because that is the multi omics problem of, ‘here are these people who have this condition. Here are all these interesting things I know about them from their EMRs. I can test all these hypotheses.’ But if I can go from testing one hypothesis a month to one hypothesis a day or an hour, that’s the promise of precision medicine.”
“The emerging trends in this area include taking AI and agentic workflows and applying them to the task of analyzing vast information. Asking AI: ‘do you see hits of anomalies that seem to correlate with a particular mechanism?’ If you do have a target, can you simulate the protein in the active site and start to perturb that and understand what may be a possible druggable area? And if you want to drug that area, how do you optimize the actual configuration and design of different molecules, and then test them for metabolic signature or toxicity, or likelihood of success in clinical trials.”
About the Author

Kara Nadeau
has 20+ years of experience as a healthcare/medical/technology writer, having served medical device and pharmaceutical manufacturers, healthcare facilities, software and service providers, non-profit organizations and industry associations.