Toward a culture shift in laboratory quality: application of the full ISO 15189 standard
In November 1999, the Institute of Medicine (IOM) published a profound article entitled “To Err Is Human: Building a Safer Health System.” The very first line of the publication reads, “Health care in the United States is not as safe as it should be—and can be.” It proceeds to say that medical errors contribute to a million injuries a year, with as many as 98,000 associated deaths.1 Emphasis is properly placed on the phrase “can be,” as one report asserts that preventable medical errors are the third-leading cause of death in America.2
The Clinical Laboratory Improvement Amendments of 1988 (CLIA '88) were established as a laboratory quality measure to ensure accuracy, reliability, and timeliness of patient results. However, clinical diagnostic laboratory error rates remain between 0.1 percent and 9.3 percent.3,4 The reduction of error rates and the implementation of systemic improvements to reduce errors are not necessarily addressed in CLIA regulations and, therefore, these measures rely on the prudence of laboratory management to develop strategic quality plans and/or procedures.
Comprehensive clinical laboratory quality management systems (QMS) are unregulated and, in many cases, are nonexistent. Along with the growing availability of waived test systems comes the reduction in quality and regulatory oversight.5 Since their inception, CLIA regulations have undoubtedly mitigated numerous occurrences of clinical errors. However, as the clinical laboratory environment changes to encompass an increasing variety of test methods, it is becoming clear that there is a need to address and regulate technical and non-technical factors that impact quality and that lead to medical errors and diminished patient care outcomes. Total eradication of medical errors is nearly impossible; however, the sources of preventable errors are typically identifiable and associated with systemic errors that can be reduced with the implementation of a sound quality system.
ISO 15189: where we are today
To date, some 60 countries have adopted ISO 15189 as a mandatory regulatory standard for clinical laboratories. While the United States contributed to the development of this standard, efforts to encourage labs to adapt to the initiative have met with much resistance—in part because compliance with only the CLIA regulations is mandatory to operate a clinical lab in the U.S. At the same time, it appears that laboratory leaders are largely aware of the ISO 15189 standard, even though they have not wholly accepted the value and validity of its comprehensive quality approach.
Overcoming the reluctance to enhance CLIA regulations with the implementation of ISO 15189 will require a cultural shift in approach to laboratory quality. If that is to occur, it is imperative that key laboratory stakeholders understand the fundamental principles of ISO 15189 and how the application of ISO 15189 can enhance the CLIA regulations.
The potential impact of ISO 15189
Among the many challenges that laboratories face, increasing laboratory quality is at the top of the list. Several principles inherent within the ISO 15189 standard are intended to address management responsibilities and performance characteristics that ultimately impact laboratory quality. Given that the vast majority of laboratory errors occur in the pre- and post-analytical phases, it is evident that the approach to improving performance in these areas is not addressed in the current laboratory regulations. The requirements of ISO 15189 focus on quality indicators throughout the laboratory, responsiveness, and involvement from top management, and continual quality improvement (Figure 1).
The result of adapting to systemic process review, modification, and improvement, as addressed in ISO 15189, is the mitigation of laboratory errors, increased quality performance, and a greater sense of confidence on the part of users of laboratory services, specifiers (private and public bodies that need accurate test data to make informed decisions), and the patient population.
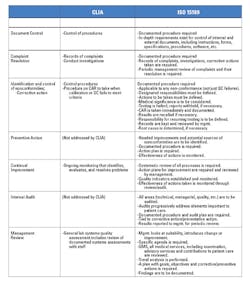
Applying both CLIA and ISO 15189
CLIA regulations serve as the sieve for laboratory quality, while the ISO 15189 standard catches quality issues that fall through that sieve—issues that are often responsible for a significant number of clinical laboratory errors. ISO 15189 delves deeper into the QMS than the CLIA standard, requiring a higher degree of quality management, the establishment of continual quality improvement, and true audit-based monitoring (Table 1).Pre- and post-analytical phase errors are preventable with systemic quality indicators. Integrating existing quality measures with a sound QMS will create a cultural shift in laboratory quality that is already prevalent in other parts of the world. When laboratories comply with CLIA and ISO 15189 requirements, they are committing to increased laboratory quality and improved patient outcomes.
______________
Changing an entrenched culture of any kind is a daunting task, yet changing the culture of laboratory medicine for the good of patient care is warranted by the ongoing incidence of laboratory errors. ISO 15189 offers laboratories a balanced, quality approach that is widely underused in the U.S. Applying both the CLIA and ISO 15189 standards, with an understanding of the way in which they complement one another, can increase overall laboratory quality, reduce systemic errors that are associated with adverse patient outcomes, and transform laboratory quality to accomplish any laboratory's goals: accuracy, reliability, and timeliness of patient results.
References
- Kohn LT, Corrigan JM, Donaldson MS (Institute of Medicine) To err is human: building a safer health system. Washington, DC: National Academy Press, 2000.
- Medical Mistakes are 3rd Leading Cause of Death in U.S. http://www.sanders.senate.gov/newsroom/press-releases/medical-mistakes-are-3rd-leading-cause-of-death-in-us. Accessed April 4, 2015.
- Commission on Office Laboratory Accreditation. http://www.cola.org/. Accessed April 4, 2015.
- Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med.2007;45:720–727.
- Federal Government Questions Quality in Waived Testing. The Hard Facts and What Can Laboratories Do Now? A COLA White Paper, 2009. http://www.cola.org/docs/waived/whitepaper.pdf. Accessed April 4, 2015.
Takeisha Farmer, MS, formerly served as Accreditation Officer for the American Association for Laboratory Accreditation (A2LA).