Since it was first described in 1948 by Hartert in Heidelberg, Germany,1 “thrombelastographie,” a method of measuring the viscoelastic properties of a blood clot during its formation, has evolved significantly. This testing method actually predates that of the activated partial thrombin time (aPTT). Originally, the thromboelastography (TEG) assay was considered interesting from a theoretical standpoint, but the clinical applicability was not well understood. Therefore, TEG was seen as being a bit too esoteric and too shock-sensitive for routine clinical use. Performing the test was also technically challenging, and the results of the TEG analysis were non-intuitive and therefore difficult for the majority of clinicians to interpret and use with confidence.
While the TEG assay experienced growth in clinical interest as new surgical procedures evolved and perioperative bleeding became more of a serious complication, it remained mainly outside the scope of conventional diagnostic application in clinical practice. Even the TEG technology itself had not significantly evolved from its original design and continued to be performed only by an astute few. Nonetheless, the TEG maintained a level of interest because it provided a unique, comprehensive way to assess hemostasis in a whole blood sample, in vitro, which more closely mirrored the physiologic conditions in vivo. Through the work of the dedicated scientific and medical community, realization of its diagnostic advantages and its clinical application began to develop.
In the early 1990s a modified thromboelastography system was developed in Munich, Germany. Calatzis et al. reported the development of an improved method of viscoelastic testing called “rotational thromboelastography” or RoTEG.2 This new method minimized many of the interferences that plagued classic thromboelastography. Later termed “rotational thromboelastometry” or “RoTEM,” the test was made simpler by automating much of the analytical process, e.g., by using an automated pipette. The sensitivity to agitation was also minimized, which allowed the device to be used in a broader range of clinical settings. The testing could now be performed more easily by clinicians outside of the laboratory setting and could deliver more reliable and reproducible results.
Thromboelastometry analytical method
Rotational thromboelastometry is performed on a stand-alone analyzer containing four testing channels and an automated pipetting system (Figure 1). Citrated whole blood samples are used in thromboelastometry to analyze the net effect of the plasma-based and cellular components on hemostasis. This demonstrates the functionality of the enzymatic factors, cofactors, and substrates involved in clot formation. Thromboelastometry analyzes clot development in vitro by measuring the increase in viscoelastic properties or clot firmness of the platelet-fibrin-clot as it is being formed. Therefore, it has been argued that thromboelastometry reflects hemostasis according to the cell-based model of coagulation from Hoffmann and Monroe more comprehensively than conventional plasmatic coagulation tests.3
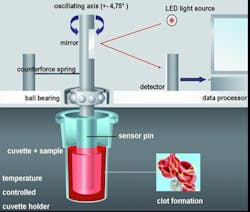
The testing procedure is performed with guided steps via automatic pipetting sequences, which minimizes pre-analytical variables by calculating reagent amounts and precisely initiates the onset of measurement. Whole blood and reagents (CaCl, activators, and additives) are combined within a small plastic cup. A plastic pin is then inserted and suspended in the cup to create a 1-mm gap between all cup and pin surfaces (Figure 2). Using a stable but sensitive ball bearing and counter spring system, a precise oscillation is induced (4.75 degrees), creating a movement between the cup and pin surfaces. As the clot forms, it imposes a slight reduction of this movement, which is detected optically by deflecting a light ray. This signal is processed by a computer system, finally displaying the results in the form of several measured parameters as well as a graph (Figure 3). Therefore, thromboelastometry provides qualitative and quantitative analysis of hemostatic function.
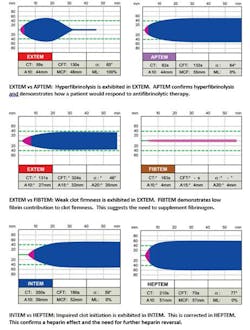
Thromboelastometry incorporates a differential approach to assessing hemostasis. By using a unique combination of activators and inhibitors, discrimination of the coagulation activity of the contact (or intrinsic) and tissue factor (or extrinsic) pathways is achieved. Activation occurs intrinsically with ellagic acid and extrinsically with tissue factor. These two activated assays serve as the basis for thromboelastometric analysis. Modifications to these two assays allow for differentiation of the causes of impaired hemostasis. Specific modifications include the addition of heparinase to the intrinsic test, which allows for assessment of enzymatic factors and for the effects of certain coagulation-altering drugs (e.g., heparin) to be elucidated. Direct fibrin clot firmness can also be rapidly determined by another modified assay. This extrinsically activated test includes the addition of a platelet inhibitor (cytochalasin D) to differentiate between hypofibrinogenemia and thrombocytopenia in bleeding patients who demonstrate a weak clot formation. Last, by comparing the base extrinsic test to another modified extrinsic test which adds an antifibrinolytic agent, hyperfibrinolysis can be confirmed, and a patient’s response to antifibrinolytic therapy can be demonstrated.
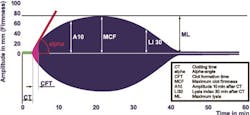
By taking a differential approach, the interpretation of results is made easier because the deficiencies in hemostasis and decisions for therapeutic intervention are made clearer. Different coagulopathic conditions create different graphic tracings (Figure 4). By considering the shapes of the thromboelastometry curves and the associated measured parameters, experienced clinicians can make a better determination of any defect of hemostasis. Clinical setting-specific (e.g. cardiovascular surgery, trauma, etc.) algorithms have been developed in order to facilitate interpretation and decision making for less experienced physicians dealing with management of severe bleeding.4,5,6
Clinical application and benefits
Clinical application. Thromboelastometry is widely used in the management of perioperative bleeding in areas such as cardiovascular surgery, orthotropic liver transplantation, postpartum hemorrhage, spinal surgery, and trauma. These clinical settings typically require therapeutic intervention involving the use of allogeneic blood products and/or coagulation factor concentrates. In patients exhibiting clinically significant bleeding, thromboelastometry can aid in determining whether the bleeding is based on a coagulopathy or is due to surgical reasons. In coagulopathic patients, impaired clot initiation, hypofibrinogenemia, fibrin polymerization disorders, thrombocytopenia, and premature clot lysis can be detected in a timely fashion by thromboelastometry. Thromboelastometry can also be used to help guide hemostatic therapy in bleeding patients in order to administer the appropriate blood products, hemostatic drugs, and coagulation factor concentrates.4,5,6 Furthermore, it provides an assessment of hypercoagulability in patients who may be at risk for thrombosis.7
The benefits of including thromboelastometry in the management of perioperative bleeding are multiple. Diagnostic performance, safety, efficacy, and cost-effectiveness have been shown to be improved using a goal-directed therapy that includes thromboelastometry as opposed to conventional laboratory testing alone to manage perioperative bleeding.
Diagnostic performance. Thromboelastometry, in combination with other laboratory assays, improves diagnostic performance by delivering more information and more timely results, which makes it relevant for helping to guide therapeutic intervention perioperatively. According to Haas et al, the turnaround time of thromboelastometry results is significantly shorter (15 to 20 minutes) than that of conventional laboratory results (45 to 60 minutes).8 Since the display provides visualization of real-time clot development, clinicians can then assess the different phases of clot development as they occur. Thromboelastometry is able to demonstrate impairment of thrombin generation due to enzymatic factor deficiency or a heparin effect within two to four minutes. Measurement of early amplitude of clot firmness (i.e, A10) allows for accurate prediction of maximum clot firmness (MCF) 9 within about 12 minutes. Rapid identification of inadequate clot firmness is critical to determine appropriate treatment of severe bleeding. The fibrin polymerization assay enables timely differential diagnosis of thrombocytopenia or hypofibrinogenemia in a bleeding patient and can aid with appropriate decision making as to whether there is a need to supplement fibrinogen or platelets.10
Along with providing faster and clearer diagnostic information, thromboelastometry allows for use without the need for special vibration-resistant surfaces due to its low shock-sensitivity. Therefore, testing can be performed in the central laboratory or in a peripheral laboratory location that best meets the individual needs of the hospital. The use of citrated whole blood samples in thromboelastometry provides for excellent sample stability of up to four hours of testing after blood sampling11 and flexibility with regard to the location in which testing can be performed. Transporting the citrated whole blood sample to a central laboratory via a pneumatic tube transport system (PTS) has been shown to be a feasible option.12 Regardless of the testing location, the analysis can be viewed as it develops real-time via a secure remote viewing system, making the results known immediately at the bedside without performing point of care testing.
Safety and Efficacy. A change in diagnostics without a change in therapeutic intervention will not result in improvement in bleeding management. Therefore, using well designed algorithms that are guided by thromboelastometry has been shown to be efficacious and can increase patient safety and improve outcomes.5,13,14 Thromboelastometric measurements have been shown to be predictive of the need for massive transfusion,15 and thromboelastometry-guided therapy can help prevent massive transfusion and improve patient outcomes by guiding hemostatic therapy and avoiding unnecessary transfusions as well as the deleterious effects of allogeneic blood transfusions.13,14 Thromboelastometry-guided therapy has also been shown to be able to predict and prevent thromboembolic events.5,13
The ability of thromboelastometry to predict bleeding and the risk of thrombosis and confirm hyperfibrinolysis and the need for activating a massive transfusion protocol (MTP) allows for faster intervention. Therefore, protocols that include thromboelastometry can then aid by stopping microvascular bleeding quickly and avoiding massive transfusion and thrombosis at the same time.5,13,14
Cost-Effectiveness. The cost of acquiring, implementing, and maintaining thromboelastometry devices may seem prohibitive in the budget-sensitive environment in which our hospital systems now must operate. However, the financial benefit that an established bleeding management program can provide to a hospital system can be significant. Thromboelastometry has been shown to be an important tool to reduce the primary costs of blood products within hospitals.5,13,16,17 In addition to primary acquisition costs of the blood products, activity-based costs of blood transfusion and secondary costs of complications related to allogeneic blood transfusion have to be considered.14,18,19 Thromboelastometry has been shown to reduce the incidence of transfusion-related adverse events13 and accordingly may reduce corresponding hospital costs. Reducing unnecessary and inappropriate allogeneic blood transfusions by implementing better bleeding management protocols will help to reduce the incidence of adverse events such as transfusion-related immunomodulation (TRIM), transfusion-associated circulatory overload (TACO), transfusion-related acute lung injury (TRALI), and thrombotic adverse events (TAEs). Reducing these secondary costs of blood transfusions may even exceed the cost-savings of reducing blood products themselves and improve patients’ clinical outcomes.
The increasing role of thromboelastometry
Clinicians are becoming increasingly educated and more mindful of implementing best practices in bleeding management and transfusion medicine. This is greatly to the benefit of their patients and economic well-being of the facilities in which they practice. Guided protocols that include thromboelastometry testing are moving closer to becoming the standard of care in the U.S. for bleeding management. The European Society of Anesthesiology has already given its grade of strong recommendation based on solid evidence that thromboelastometry should be included in perioperative bleeding management protocols.20
No one device can meet every challenge that is presented to the physician faced with managing severely bleeding patients. Every diagnostic device on the market has inherent and unique limitations, and it is important to understand these limitations for the best clinical application of the technology. Tanaka et al have reported limitations of non-modified viscoelastic testing.21 Medical technology industry leaders are poised to address some of these diagnostic gaps and inherent limitations with the development of new technology and additional assays. With the continued development of new anti-thrombotic drugs and anticoagulant therapies, this becomes more challenging to manage on one platform.22,23
As such, the results from thromboelastometric analysis should not be the sole basis for a patient diagnosis. These results should be considered along with a clinical assessment of the patient’s condition the clinical setting and other laboratory tests. It is therefore imperative to pair them with complementary diagnostic technology, e.g., thromboelastometry and platelet aggregometry devices, in order to adequately meet the diagnostic and therapeutic challenges that are inherent in hemostasis management.4,13,14,21,24
References
- Hartert H. Blutgerinnungsstudien mit der Thrombelastographie, einem neuen Untersuchungsverfahren. Klinische Wochenschrift. 1948;26(37/38):577-583.
- Calatzis A, Hipp R, Mössmer G, Stemberger A. The roTEG coagulation analyser: a new thrombelastographic system for whole blood coagulation analysis. Int. Congr. Clin. Chem. 1996; abstract B610.
- Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85(6):958-965.
- Görlinger K. Coagulation management during liver transplantation. Hämostaseologie. 2006;26(3 Suppl 1):S64-76.
- Görlinger K, Dirkmann D, Hanke AA, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115(6):1179-1191.
- Schöchl H, Maegele M, Solomon C, Görlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;24(20):15.
- Dimitrova-Karamfilova A, Patokova Y, Solarova T, Petrova I, Natchev G. Rotation thromboelastography for assessment of hypercoagulation and thrombosis in patients with cardiovascular diseases. J Life Science, 2012;6:28-35.
- Haas T, Spielmann N, Mauch J, Speer O, Schmugge M, Weiss M. Reproducibility of thrombelastometry: Point-of-care versus hospital laboratory performance. Scand J Clin Lab Invest. 2012;72(4):313-317.
- Görlinger K, Dirkmann D, Solomon C, Hanke AA. Fast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulability. Br J Anaesth. 2013;110(2):222-230.
- Larsen OH, Fenger-Eriksen C, Christiansen K, Ingerslev J, Sørensen B. Diagnostic performance and therapeutic consequence of thromboelastometry activated by kaolin versus a panel of specific reagents. Anesthesiology. 2011;115(2):294-302.
- Theusinger OM, Nürnberg J, Asmis LM, Seifert B, Spahn DR.Rotation thromboelastometry stability and reproducibility over time. Eur J Cardiothorac Surg. 2010; 37(3):677-683.
- Lancé MD, Kuiper GJ, Sloep M, et al. The effects of pneumatic tube system transport on ROTEM analysis and contact activation assessed by thrombin generation test. Thromb Res. 2012;130(3):e147-150.
- Weber CF, Görlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117(3):531-547.
- Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet. 2013; 381(9880):1855-1865.
- Schöchl H, Cotton B, Inaba K, et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit Care. 2011;15(6):R265.
- Anderson, L, Quasim I, Soutar R, Steven M, Macfie A, Korte W. An Audit of Red Cell and Blood Product Use After the Institution of Thromboelastometry in a Cardiac Intensive Care Unit. Transfus Med. 2006;16(1):31-39.
- Spalding, GJ, Hartrumpf M, Sierig T, Oesberg N, Kirschke CG, Albes JM. Cost reduction of perioperative coagulation management in cardiac surgery: value of ‘bedside’ thrombelastography (ROTEM). Eur Cardiothorac Surg. 2007;31(6):1052-1057.
- Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010; 50(4):753-765.
- Hofmann A, Ozawa S, Farrugia A, Farmer SL, Shander A. Economic considerations on transfusion medicine and patient blood management. Best Pract Res Clin Anaesthesiol. 2013;27(1):59-68.
- Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270-382.
- Tanaka KA, Bader SO, Sturgil EL. Diagnosis of perioperative coagulopathy—plasma versus whole blood testing. J Cardiothorac Vasc Anesth. 2013;27(4 Suppl):S9-15.
- Schaden E, Schober A, Hacker S, Kozek-Langenecker S. Ecarin modified rotational thrombelastometry: a point-of-care applicable alternative to monitor the direct thrombin inhibitor argatroban. Wien Klin Wochenschr. 2013;125(5-6):156-159.
- Körber MK, Langer E, Ziemer S, Perzborn E, Gericke C, von Heymann C. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost. 2013. [Epub ahead of print]
- Görlinger K, Shore-Lesserson L, Dirkmann D, Hanke AA, Rahe-Meyer N, Tanaka KA. Management of hemorrhage in cardiothoracic surgery. J Cardiothorac Vasc Anesth. 2013;(4 Suppl):S20-34.