Interest in vitamin D testing has grown exponentially during recent years. Several publications link vitamin D to a range of issues related to cardiology, cancer, pregnancy, metabolic disorders, mortality, and so on. Vitamin D testing creates opportunities, but also challenges, for the clinical laboratory.1
25-OH Vitamin D is a challenging analyte to accurately measure in human blood due to the presence of 25-OH Vitamin D2, Vitamin D binding proteins, 25-OH Vitamin D metabolites such as 24, 25 (OH)2 Vitamin D, and C-3 epimers of 25-OH Vitamin D.1 Variability between methods due to lack of standardization has added to the challenges.
As new immunoassays for Vitamin D testing are released, laboratorians need to look carefully at the following factors to determine whether a given assay is a good fit for their laboratory and their patient population.
1. Equimolar detection of 25-OH Vitamin D3 and D2
25-OH vitamin D immunoassays are required to detect 25-OH Vitamin D2 and 25-OH Vitamin D3 in an equimolar fashion and report a total 25-OH Vitamin D result.2
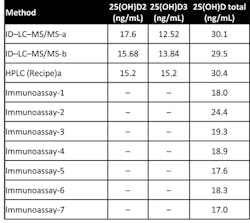
Some studies have shown that many immunoassays do not completely measure 25-OH Vitamin D2 and that many assays are unable to reliably measure samples containing 25-OH Vitamin D2. External quality assurance programs, such as Vitamin D External Quality Assessment Scheme (DEQAS), and various publications have demonstrated that some immunoassays underestimate 25-OH Vitamin D because they do not measure 25-OH Vitamin D2 and D3 in an equimolar fashion. One example is sample 390 from the DEQAS, in which the LC-MS/MS methods measured 25-OH Vitamin D2 completely and most immunoassays did not measure the 25-OH Vitamin D2. Since the specimen was at the clinical decision limit of 30 ng/mL, the immunoassay specimens would be classified as vitamin D insufficient/deficient (Table 1).
2. Clinical decision limits for 25-OH Vitamin D
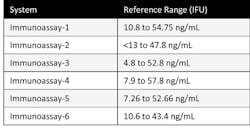
According to the Endocrine Society’s clinical guidelines, vitamin D deficiency is defined as a 25(OH)D below 20 ng/ml (50 nmol/liter) and vitamin D insufficiency as a 25(OH)D of 21–29 ng/ml (52.5–72.5 nmol/liter).3 Based on these guidelines, physicians use the range of 30 to 100 ng/mL as sufficient vitamin D levels irrespective of the manufacturers’ established ranges. Some of the manufacturer-established reference ranges for different immunoassays are shown in Table 2.
Since the ranges in the guidelines were established using the LC-MS/MS or RIA methods for Vitamin D which detect equimolar 25-OH Vitamin D3 and D2, it becomes important for laboratories to determine if the immunoassay of their choice aligns to these methods. If an assay does not align to these methods, then it’s important that the laboratory consider using a manufacturer specific reference range to avoid underestimating 25-OH Vitamin D, because the assay may not detect 25-OH Vitamin D2 accurately.
3. Lack of harmonization among LC-MS/MS methods
One significant challenge is that not all liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods are harmonized.4,5 Each LC-MS/MS method may be aligned to a different reference standard and hence provide variable results. The introduction of the NIST SRM 972 has improved the comparability of LC-MS/MS methods, but the external quality assessment programs have shown that there is substantial variability in these methods. Physicians sending specimens to reference laboratories have to be aware of this variability. Inquiring about the DEQAS results and reference standard used will provide meaningful information that they will need before making clinical decisions.
4. NIST SRM 972
The NIST standard reference material (SRM) 972 is available as a reference standard. Four levels are available:
Level 1 has been prepared from human serum and is unaltered. The LC-MS/MS methods and certain immunoassays reached the certified value within 10% deviation.
Level 2 was prepared by diluting level 1 with horse serum to achieve a lower 25-OH Vitamin D concentration. LC-MS/MS methods agreed with the certified values.
Level 3 contains human serum that has been fortified with 25-OH Vitamin D2. LC-MS/MS methods showed complete detection of 25-OH Vitamin D2 in this sample. However, most of the immunoassay methods could only partially detect this compound.
Level 4 contains human serum that has been fortified with 3-epi-25 OH Vitamin D3. This compound was completely detected by LC-MS/MS methods and some immunoassays. Dr. Linda Thienpont, PhD, at Ghent University, has developed an ID-LC-MS/MS method for vitamin D in human serum that is traceable to NIST SRM 972 and that separates the 3-epi-25-OH Vitamin D3 from 25-OH Vitamin D3.6
5. Cross reactivity to 3-epi-25 OH Vitamin D3
Since LC-MS/MS methods and some immunoassays measure 3-epi 25 OH Vitamin D, many researchers have looked into whether the presence of this compound will affect routine measurement of vitamin D.
Keevil and colleagues have estimated that at the vitamin D deficiency level of 50 nmol/L (20 ng/mL), the concentration of 3-epi-25 OH Vitamin D3 would equate to 2.5 nmol/L (1ng/mL), which would not significantly influence the clinical interpretation in either children or adults.7
As it seems unlikely that the vitamin D sufficiency/insufficiency status of an individual would be altered by the presence of the epimer, it is not necessary to quantify the epimer in these subjects. Therefore, a two-phase testing system may be beneficial where total 25-OH Vitamin D is first quantified, and subsequently, in borderline subjects 3-epi-25 OH D3 is quantified. This approach may be particularly indicated in the case of infants.8
6. Cross reactivity to 24, 25 (OH)2 vitamin D
Unlike the 3-epi-25 OH Vitamin D3, the levels of 24, 25 (OH)2 Vitamin D in serum may be significant enough to affect the clinical decision. The levels of 24, 25 (OH)2 Vitamin D in normal adult serum can be up to 13.5 nmol/L (5.4 ng/mL).9 This can be significant enough to affect sufficiency/insufficiency status of an individual.
7. Linearity or measuring range of immunoassays
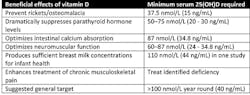
Hypovitaminosis D (Low 25-OH Vitamin D) has been classified as:10
- Insufficient 50–100 nmol/L (20–40 ng/mL)
- Mild 25–50 nmol/L (10–20 ng/mL)
- Moderate 12.5–25.0 nmol/L (5–10 ng/mL)
- Severe < 12.5 nmol/L (< 5 ng/mL)
These levels have been classified based on the levels of 25-OH Vitamin D producing health benefit (Table 3).10
Many immunoassays do not meet the lower limit of detection of
8. The Vitamin D Standardization Program
The Centers for Disease Control and Prevention (CDC) has introduced a vitamin D Standardization-Certification Program to ensure reliable protocols for standardizing procedures to measure 25-hydroxyvitamin D. It also aims to improve public health practice by promoting measurements that are accurate and comparable over time, across locations, and independent of laboratory methods.
9. Steps that need to be taken after a laboratory has decided to start testing for 25-OH vitamin D
After taking all the factors discussed above into consideration, a laboratory needs to complete certain validations before reporting results on the new 25-OH Vitamin D method:
- All laboratory personnel must be aware of the method comparison study before bringing in a new test. While the regression analysis is an important step, laboratories need to pay attention to the medical decision limits and how the new method affects clinical decisions.
- If the laboratory is sending specimens to more than one reference laboratory, care should be taken so that the data points from different methods are not combined. For example reference lab-1 may use LC-MS/MS and reference lab-2 may use an immunoassay method that does not detect 25-OH D2 completely.
- Specimen collection and stability are important factors for testing 25-OH Vitamin D. Laboratories should pay careful attention to the specimen types, collection tubes, and stability information provided by the manufacturer. For example specimens collected in serum separator tubes, and not separated immediately, may show > 10% variation in results. This can affect the clinical decision.
- Hemodialysis specimens show interference in some immunoassays, and care should be taken when reporting results from certain immunoassay manufacturers.
With the Vitamin D standardization program, it can be expected that immunoassay manufacturers will make modifications to existing assays. Pay careful attention to changes in your immunoassay method and contact the manufacturer if you have any concerns.
References
- Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012;58(3):531-542.
- Herrmann M. The measurement of 25-hydroxy vitamin D–an analytical challenge. Clin Chem Lab Med. 2012;50(11):1873-1875.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline, J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- Tai SS, Bedner M, Phinney KW. Development of a candidate reference measurement procedure for the determination of 25- hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82(5):1942-1948.
- Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotopedilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57(3):441-448.
- Thienpont LM, Stepman H, Vesper HW. Standardization of measurements of 25-hydroxyvitamin D3 and D2. Scand J Clin Lab Invest. 2012;72(Suppl 243):41-49.
- Keevil B. Does the presence of 3-epi-25OHD3 affect the routine measurement of vitamin D using liquid chromatography tandem mass spectrometry? Clin Chem Lab Med. 2011;50:181-183.
- Bailey D, Veljkovic K, Yazdanpanah M, Adeli K. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin Biochem. 2013;46:190-196.
- Coldwell RD, Trafford DJ, Makin HL, Varley MJ, Kirk DN. Specific estimation of 24,25-dihydroxyvitamin D in plasma by gas chromatography-mass spectrometry. Clin Chem. 1984;30(7):1193-1198.
- Stroud ML, Stilgoe S, Stott VE, Alhabian O, Salman K. Vitamin D–a review. Aust Fam Physician. 2008;37(12):1002–1005.