Of the methods employed in today’s molecular diagnostic laboratory, none is more pervasive than the polymerase chain reaction (PCR). An understanding of its basic principles is crucial to an understanding of molecular diagnostics.
In essence, PCR is a technique to amplify a short, predefined sequence of DNA present in a sample from a very low number of copies (possibly a single copy), to very large (and thus more readily detectable) numbers of sequence-identical copies. The method itself is simple and builds on the concepts of nucleic acid structure, strand denaturation and annealing, and DNA polymerase activity as covered in previous installments of this series.
To understand the process, let’s first consider what components go into the PCR tube:
- an aqueous buffer, containing Mg2+ ions and a pH buffering agent plus (sometimes) other particular cofactors which aid the polymerase to be employed;
- dNTPs, as the building blocks of nascent DNA strands by the DNA polymerase;
- thermostable DNA polymerase (that is, one not inactivated by heating);
- a pair of short, defined-sequence, single-strand synthetic DNA oligonucleotide primers. Two aspects of these primers are critical for the process: first, they must be complementary to two nearby sections of the target sequence to be amplified (generally for diagnostic applications, on the order of 100 to 500 base pairs apart). Second, the primers must be complementary to opposite strands of their target sequence, and oriented such that each primer when annealed to its complementary sequence points its 3′ end towards the location of the other primer’s sequence. Each primer of the pair is present in very large numbers in the reaction tube.
- a sample containing DNA among which the target sequence template exists.
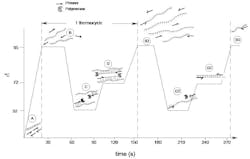
Imagine a microcentrifuge tube containing all of the above, placed in a programmable temperature control instrument (a thermocycler) and subjected to the temperature-vs-time profile shown in Figure 1.
As the process starts at (A), our sample template is in its native, double-stranded form as shown. Raising the temperature to 95°C (denaturation; B) will cause our template molecule to denature to free single DNA strands. The reaction is now rapidly cooled to a temperature at or slightly below the annealing temperature or TM of our primers (here, for the sake of example, being 52°C) (annealing, C). The rapidity with which this cooling occurs and the vast molar excess of primer relative to template strands means that it is much more likely that the primers will anneal to their complementary regions on the template than the two longer template molecules will anneal to each other.
In light of previous sections of this series, the reader will now recognize that the structures present at (C) represent substrates for DNA polymerase to work on. Being thermostable, the polymerase present in our reaction was not damaged by the high-temperature denaturation (B). But neither was it tremendously active at this lower temperature, which thus acts only to attach to the 3′ end of each annealed primer on its template molecule. It remains to raise the temperature to one more conducive to enzyme activity, 72°C, (extension; D) and allow the polymerases to act on their substrates, extending the 3′ end of each primer in a template-directed fashion toward the opposing primer’s sequence. Each polymerase is thus creating a new copy of one strand of the target sequence.
How long does this extension proceed? Again, the reader will recall that DNA polymerase activity will proceed until the end of a template is reached, or the reaction is stopped by outside forces. Here, the latter will occur by the thermocycler program again raising the temperature to a denaturation step (B2), causing polymerase dissociation from the growing new strand and denaturation of all DNA strands. What is critical here is that the time allowed at the extension phase (D) be somewhat longer than the polymerase will take, to extend a growing strand from its primer to the site of the opposing primer sequence. As a rule of thumb, one minute per 1000 base pairs is commonly used, meaning that extension times in diagnostic PCR reactions are often less than 30 seconds.
Steps B, C, and D constituted one thermocycle of our PCR reaction. Now consider what occurs in a subsequent cycle, and focus your attention on the new DNA strands produced in the first cycle. Each of these strands is fairly short, begins with one of the primer sequences which initiated its growth, and contains (somewhere near its 3′ end) a sequence complementary to the opposing primer of the primer pair. As the second PCR cycle cools to annealing, each of these new strands thus can serve as an annealing partner for the opposing primer, and recruit in a polymerase (shown at C2); when raised to extension temperature, the polymerase extends this new annealed primer (D2). Notice something here, however: unlike in the first cycle, where polymerase activity was terminated by raising the reaction temperature, here the polymerase will run out of template strand before the temperature is raised. We have in fact now created a DNA strand which is exactly defined at each end by the two primers of the set (B3).
The amplification power of PCR arises from the fact that as we now proceed into yet more cycles, there will be an exponential increase in the number of these exact, primer–to–primer amplicon molecules (for with each cycle, an amplicon can denature to two strands, each of which is replicated, thus generating 2N amplicon molecules for N thermocycles). By contrast, only a linear increase (2xN) in number of the uncertain length products as made in the first cycle occurs. While proving this is beyond the available space of this article, inquisitive readers wishing to prove this for themselves could draw out the first few cycles of a PCR reaction. By the fourth cycle the truth of the above statement will be observed. In practice, by 40 thermocycles of an ideal PCR reaction, a single template molecule can be converted into 240 or approximately 1×1012 amplicon copies, and by this stage reagent depletion (that is, the using up of dNTPs and primers) occurs and generally limits the utility of further thermocycling.
Here are key aspects of this entire PCR process as described:Only simple reagents were needed, and all could be placed in the tube at the outset of the reaction.
- Different chemistries (denaturation, annealing, extension) can be driven purely by easily automated, external forces on the reaction tube (via the thermocycler).
- Reagents for PCR are generic except for the target-specific primer set.
- Individual thermocycles are short, on the order of 90 seconds or less.
- The presence of the intended target sequence, as defined by two regions complementary to the primers, results in production of large numbers of a defined DNA product.
- Conversely, a lack of presence of the intended target sequence should result in a lack of product formation, as the primers lack a template for productive annealing and subsequent primer extension.
When coupled to a method for the detection of the reaction specific products (to be covered in subsequent installments of this series), PCR thus offers a rapid, simple, generic, highly sensitive, and highly specific test for the presence or absence of any defined DNA sequence. In the context of our definition of molecular diagnostics, PCR thus provides an indispensable tool.
How is this process adjusted when our nucleic acid of interest is RNA, not DNA? Without going into excessive detail, suffice it to say we call upon an enzyme, reverse transcriptase, drawn from the genome of certain classes of viruses. This enzyme is in effect a DNA polymerase, but with ability to use an RNA strand as its template for extension of an annealed primer. While reverse transcriptases are not themselves robust enough to survive the multiple thermocycles of a PCR reaction, they can be included at the outset and where a primer of the PCR reaction is complementary to a section of an RNA template molecule. Preincubation of the reaction at a moderate temperature (37°C to perhaps 55°C) will allow it to create a single-strand DNA molecule copy (a “cDNA”) of the original RNA target. This cDNA can then be processed exactly as a regular PCR reaction. In many modern RT-PCR (Reverse Transcription–PCR) reaction formulations, buffers have been optimized to allow both the reverse transcriptase and subsequent DNA polymerase steps to occur without any intervening changes or additions to the reaction, making RT-PCR in practice very nearly as simple as plain PCR.
About the Author