A clinical trial from Keck Medicine of USC will test the feasibility of treating non-small cell lung cancer with immunotherapy provided at home.
The study will examine if a new formulation of atezolizumab, an immunotherapy approved for treating certain types of non-small cell lung cancer, can instead be safely and effectively administered subcutaneously, meaning it is injected under the patient’s skin, by a nurse at the patient’s home, along with telemedicine appointments and remote monitoring through wearable trackers.
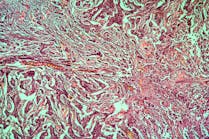
The drug used in the clinical trial, atezolizumab, consists of a monoclonal antibody, a man-made protein that triggers the immune system to attack cancer cells. It was approved by the Food and Drug Administration in 2016 for late-stage lung cancer and more recently in 2021, for early stage, non-small cell lung cancer.
Investigators aim to enroll 37 patients with non-small cell lung cancer who are deemed eligible to receive immunotherapy as treatment. Patients will receive treatments administered by a nurse who will visit their home every three weeks for one to two years.
Additionally, the researchers will use the latest digital tools to track patients’ vital signs, physical activity and other markers of health remotely and monitor patients with telehealth visits.
The study will not only examine the feasibility of home administration of the medication, but how well patients comply with the program and how satisfied they are with being treated remotely and through telehealth appointments.
Keck Medicine of USC release on Newswise