Common inherited genetic factors that predict cancer risk in the general population may also predict elevated risk of new cancers among childhood cancer survivors, according to a study led by researchers at the National Cancer Institute (NCI), part of the National Institutes of Health.
The findings, published in Nature Medicine, provide additional evidence that genetics may play an important role in the development of subsequent cancers in survivors of childhood cancer and suggest that common inherited variants could potentially inform screening and long-term follow-up of those at greatest risk.
Childhood cancer survivors are known to have a higher risk of developing a new cancer later in life due to adverse effects of cancer treatment or rare inherited genetic factors. In the new study, the researchers evaluated the combined effect of common variants with history of radiation treatment and found the resulting elevated cancer risk was greater than the sum of the individual associations for treatment and genetic factors alone.
To assess the contribution of common inherited genetic variants to risk of subsequent cancer in people who survived childhood cancer, the research team used data from genome-wide association studies, or GWAS, that had been conducted in large populations of healthy individuals. Such studies have identified thousands of common inherited variants associated with risk of different cancers. The risk associated with a single common variant is typically small, but the effects of large numbers of variants can be bundled into a summary score, or polygenic risk score, that provides a more comprehensive estimate of someone’s genetic risk.
Although polygenic risk scores have shown promise for predicting cancer risk in the general population, it has not been known whether such scores are also associated with the risk of subsequent cancer among childhood cancer survivors.
To find out, the researchers looked at the association between polygenic risk scores and risk of basal cell carcinoma, female breast cancer, thyroid cancer, squamous cell carcinoma, melanoma, and colorectal cancer among 11,220 childhood cancer survivors from two large cohort studies. For all of these cancers except colorectal cancer, polygenic risk scores derived from GWAS in the general population were associated with the risk of these same cancers among childhood cancer survivors.
The researchers then looked at basal cell carcinoma, breast cancer, and thyroid cancer — malignancies that occurred most often in the combined data set and that are strongly linked to radiation therapy — to examine the joint effect of polygenic risk score and treatment history. They found that risk associated with the combination of higher-dose radiation exposure and higher polygenic risk score was greater than would be expected based on simply adding the risk associations of each individual risk factor.
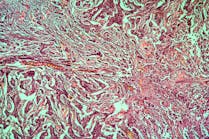
For basal cell carcinoma, a high polygenic risk score was associated with 2.7-fold increased risk compared with a low polygenic risk score among survivors. History of higher radiation exposure to the skin was associated with a 12-fold increase in risk, compared with lower radiation exposure to the skin. However, survivors with high polygenic risk scores and higher doses of radiation to the skin had an 18.3-fold increased risk of basal cell carcinoma, compared with those with low polygenic risk scores who had received lower radiation doses to the skin.
Moreover, by age 50, survivors with higher polygenic risk scores and higher radiation exposure had a greater cumulative incidence of basal cell carcinoma, breast cancer, or thyroid cancer than those with lower polygenic risk scores or lower radiation exposure. For example, among female survivors who had radiation to the chest, 33.9% of those with a high polygenic risk score had been diagnosed with breast cancer by age 50, compared with 21.4% of those with a low polygenic risk score.