The dramatic decline in cervical cancer in women is attributable first to screening with the Papanicolaou (Pap) test, followed later by the addition of the human papilloma virus (HPV) test, which enhanced screening sensitivity.1,2 In keeping with this excellent performance record, the current standard of care for cervical cancer screening for most women (those over 30), as currently recommended by U.S. guidelines, is cotesting with Pap and HPV tests.3,4 In 2014, the Food and Drug Administration (FDA) approved an HPV assay to be used alone as a primary screening test,5 initiating a debate about what is the best way to detect cancer.
Since this FDA decision, a retrospective, cross-sectional analysis performed in 256,648 women demonstrated that HPV-alone testing missed 18.6 percent of 526 cervical cancer cases detected by cotesting.6 The challenge is to improve screening cost-effectiveness without compromising efficacy, and the notion that screening with one test may be more cost-effective than two tests seems reasonable upon first consideration—but closer examination reveals this assumption to be wrong. Understanding the cost-benefit ratio of screening methods requires a thorough analysis of HPV assay technologies, what they detect, and how these factors influence the cost-effectiveness of cervical cancer screening.
Assays for HPV detection
Not all HPV assays are the same, and the first step in understanding their differences is to review the relationship between HPV and cervical cancer. Cervical cancer is predominantly caused by a small group of 14 genetically related high-risk (hr) HPV species, and more than 70 percent of cervical cancers are caused by the hrHPV 16 and 18 genotypes.2 The majority of hrHPV infections spontaneously regress; however, persistent hrHPV infection and expression of the E6 and E7 oncoproteins are associated with neoplastic transformation. The hurdle that must be overcome to improve cervical cancer screening cost-effectiveness is distinguishing the minority of HPV infections or lesions prone to progression from the vast majority likely to regress. Improving the ability to make this distinction would limit unnecessary follow-up costs.
There are currently five nucleic acid-based hrHPV assays available in the U.S. for cervical cancer screening.7,8 Four of the assays analyze HPV DNA; one analyzes HPV messenger RNA (mRNA). By detecting HPV mRNA, as opposed to DNA, this latter test identifies expression of HPV E6 and E7 oncoproteins.
Comparing specificity
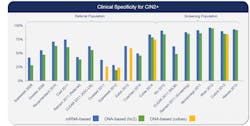
Numerous studies have demonstrated that the mRNA-based hrHPV assay has higher clinical specificity for detection of cervical cancer lesions than DNA-based hrHPV assays8-10 (Figure 1). The underlying explanation for improved specificity is detection of mRNA encoding E6 and E7 oncogenic proteins indicating lesion progression to invasive cancer. When E6 and E7 proteins are detected, as opposed to HPV DNA detection indicating viral presence only, fewer false positive test results have been reported. A recent study by Reid et al. demonstrated a 24 percent reduction in false positive test results with mRNA-based compared with DNA-based hrHPV assays.11 Whether mRNA-based hrHPV testing performs comparably to DNA-based hrHPV testing in a cervical cancer screening protocol was also explored in this study.
Comparing sensitivity
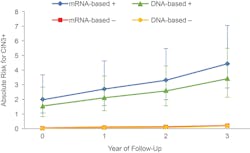
The Reid study compared the clinical performance of DNA-based hrHPV assays and an mRNA-based hrHPV assay using a cotest strategy for cervical cancer screening in a cohort of 10,860 women aged 30 years or older.11 The cumulative three-year absolute risk for CIN3 (cervical intraepithelial neoplasia) or higher was comparably low for either test for women with a negative result, indicating both tests provide a high negative predictive value (Figure 2). In contrast, and consistent with current U.S. cervical cancer screening,12 a similar significantly greater risk of CIN3 or higher with either test was observed for women with positive results. Together these data, and other studies,8-10 indicate that cotesting with mRNA-based compared with DNA-based hrHPV assays delivers equivalent clinical sensitivity, but mRNA-based testing was more specific for detection of CIN2 or worse.
Comparing cost-effectiveness
The impact of the improved specificity on cost-effectiveness of cervical cancer screening was investigated in a study by Ting et al.13 that compared costs and health outcomes between mRNA-based and DNA-based hrHPV assays in the context of current U.S. cervical cancer screening guidelines. Screening efficiency was examined using two different screening strategies: cotesting with HPV and liquid-based cytology in women 30 to 65 years; and triage of women with mild cervical cytological abnormalities (ASC-US) in the United States.
A Markov model for stochastic cost-effectiveness analysis was constructed using data from the Reid study and another by Monsenego et al.,13-15 both conducted in population-based settings. The model followed a theoretical cohort of women from age 12 to 100 years, not vaccinated for HPV, and assumed that at the beginning of the simulation that no woman was infected with HPV, or had CIN or cancer.
For both cotesting and ASC-US triage screening protocols, mRNA-based hrHPV testing cost less than DNA-based hrHPV testing; however, differences between the protocols were not statistically significant.13 To better estimate cost-effectiveness differences, an analysis of willingness-to-pay thresholds was performed. Results indicated a 100 percent probability that DNA-based testing was not cost-effective, relative to mRNA-based testing, at the $100,000 per life-year saved threshold for ASC-US triage, and a 50 percent probability that DNA-based testing was not cost-effective at the $100,000 per life-year saved threshold for cotesting.
Preliminary results from another study16,17 comparing cost-effectiveness between cotesting with an mRNA-based hrHPV assay and a DNA-based hrHPV assay alone found that mRNA-based cotesting (combined with follow-up genotyping) provided improved clinical and economic outcomes.
These studies show cervical cancer screening efficiency can be affected by the type of HPV assay used as well as the overall strategy. Results from these studies provide evidence that cotesting with an mRNA-based hrHPV assay is more likely to be cost-effective than DNA-based hrHPV testing, whether DNA-based hrHPV testing was used in cotesting or alone as a primary screening strategy.
It is worth noting that DNA-based primary screening has not been recommended by screening guidelines at this time. The cost-benefit associated with the mRNA-based hrHPV assay observed in these analyses arises from the improved specificity of the mRNA-based assay that detects expression of HPV oncogenic proteins, as opposed to DNA-based testing that reports only presence of the virus. The ability to detect expression of HPV oncogenic proteins favors the distinction between HPV infections that will progress to cervical cancer from those that will regress, leading to fewer cases where HPV nucleic acids are detected but disease is not present. The information provided by an mRNA-based hrHPV assay result gives healthcare providers and patients greater assurance about whether or not there is a need for follow-up procedures. The improved specificity of mRNA-based hrHPV assays, together with improved sensitivity of mRNA-based hrHPV assay cotesting compared with DNA-based hrHPV testing, suggest transitioning to DNA-based hrHPV testing alone as a primary screening would be not only less efficacious, but also more costly.
REFERENCES
- GLOBOCAN Cancer Fact Sheet. Cervical cancer estimated incidence, mortality, and prevalence worldwide in 2012. http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp.
- Schiffman M, Solomon D. Clinical practice. Cervical-cancer screening with human papillomavirus and cytologic cotesting. N Engl J Med. 2013;369:2324-2331.
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16(3):175-204.
- Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. 2012 ASCCP Consensus Guidelines Conference. Obstet Gynecol. 2013;121(4):829-846.
- Laine S. FDA approves first human papillomavirus test for primary cervical cancer screening http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm394773.htm Published April 24, 2014.
- Blatt AJ, Kennedy R, Luff RD, Austin RM, Rabin DS. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123(5):282-288.
- The five FDA-approved HPV assays. MLO. 2012;44(7):14-18.
- Luhn P, Wentzensen N. HPV-based tests for cervical cancer screening and management of cervical disease. Curr Obstet Gynecol Rep. 2013;1(2):76-85.
- Haedicke J, Iftner T. A review of the clinical performance of the Aptima HPV assay. J Clin Virol. 2015, Nov 6.
- Iftner T, Becker S, Neis KJ, et al. Head-to-head comparison of the RNA-based Aptima human papillomavirus (HPV) assay and the DNA-based hybrid capture 2 HPVtest in a routine screening population of women aged 30 to 60 years in Germany. J Clin Microbiol. 2015;53(8):2509-2516.
- Reid JL, Wright TC Jr, Stoler MH, et al. Human papillomavirus oncogenic mRNA testing for cervical cancer screening: baseline and longitudinal results from the CLEAR study. Am J Clin Pathol. 2015;144(3):473-483.
- Gage JC, Schiffman M, Katki HA. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J Natl Cancer Inst. 2014;106(8).
- Ting J, Smith JS, Myers ER. Cost-effectiveness of high-risk human papillomavirus testing with messenger RNA versus DNA under United States guidelines for cervical cancer screening. J Low Genit Tract Dis. 2015;19(4):333-339.
- Castle PE, Eaton B, Reid J, Getman D, Dockter J. Comparison of human papillomavirus detection by Aptima HPV and cobas HPV tests in a population of women referred for colposcopy following detection of atypical squamous cells of undetermined significance by Pap cytology. J Clin Microbiol. 2015;53(4):1277-1281.
- Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjänen K, et al. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening: the FASE study. Int J Cancer. 2011;129(3):691-701.
- Felix JC, Lacey MJ, Miller JD, Lenhart GM, Kulkarni R. Cost-effectiveness of DNA and mRNA based cervical cancer screening strategies: Preliminary results from an economic modeling analysis. Presented at 30th International Papillomavirus Conference and Clinical Workshop Lisbon, Portugal September 17-21, 2015.
- Felix JC, Lacey MJ, Miller JD, Lenhart GM, Kulkarni R. Clinical-economic modeling analysis of human papillomavirus (HPV) cotesting with genotyping versus primary HPV testing for cervical cancer screening. Value Health. 2015;18(7):A353.
Editor’s note: Please note that some of the studies cited in this article were sponsored by the manufacturer of the mRNA-based hrHPV assay to which the article alludes.