Mass spectrometry allows up to one million times resolution
Mass spectrometry can help identify what things are made of, being able to identify small molecules metabolized in the body, which helps researchers with everything from drug metabolization to how foods break down within the body and more. The silver lining of the COVID-19 pandemic was that it opened more avenues of research, shining a new spotlight onto mass spectrometry, which combines concepts from physics and chemistry to advance the medical field.
Mitch Kennedy, President of Chromatography and Mass Spectrometry at Thermo Fisher Scientific, hosted a webinar about mass spectrometry advances,1 explaining the latest advances in mass spectrometry are not only in the healthcare field, but also in food, environmental, water testing, and testing the contamination in lithium ion batteries. These open vast new areas of research, including how carbohydrates break down in food, enhancing diet and nutrition.
Mass spectrometry allows up to one million times resolution, revealing complex molecular structures. Kennedy mentioned when a drug is manufactured, the U.S. Food and Drug Administration (FDA) requires manufacturers to identify the primary and secondary metabolites, the pieces the body metabolizes from the product, which mass spectrometry can identify.
From metabolites to lipids, this helps find molecules in the body, as mass spectrometry deconvolutes to see what is present. First used to measure the mass of atoms, it proved the existence of isotopes, which inspired atomic structure.
History of mass spectrometry
Mass spectrometry harkens back to the hunt for the electron.2 J. J. Thomson first measured electrons in 1897. Credited at being the inventor of mass spectrometry, even though he was looking for something else, he received the 1906 Novel Prize in Physics for discovering the electron.
His protégé Francis Aston, who won the 1922 Nobel Prize in Chemistry, built the first mass spectrometer to measure the masses of charged atoms. For a few decades, Aston and others redesigned and improved resolution to separate isotopes. As these isotopes were important during World War II’s Manhattan Project, mass spectrometry became more popular.2
Alfred Nier perfected the usability of mass spectrometry, including producing a design with E.G. Johnson called the Nier-Johnson mass spectrometer. Enrico Fermi prompted Nier to attempt separation of uranium isotopes by mass spectrometry, which revealed which isotope was responsible for slow neutron fission, resulting in the nuclear age.
Chemists pushed this field further, as they sought to find how much of what element is in specific mixtures and chemical compounds, such as industrial chemists measuring concentrations. Fred McLafferty, Klaus Biemann and Carl Djerassi fragmented organic molecules, displaying how fellow chemists could determine molecular structure, including the structure of steroids. Research continued to expand to all the ions measured at once, instead of varying the magnetic field to get better resolution. The 1980’s advanced analysis of proteins, nucleic acids and complex carbohydrates.
The advances in the field have since been tremendous, as it was only in 2008 that researchers were discovering haploid (one set of chromosomes) versus diploid (two sets of chromosomes) cells, and in 2011, researchers could see to a depth of 10,000 proteins. In 2013, the advances continue as researchers studied an inactive X chromosome (Xi) for interactions, such as cohesion and repulsion, demonstrating the role of how RNA directs chromosomes, and in 2018, the high-fidelity mass analysis showed heterogeneity in intact ribosomal particles, revolutionizing the role of ribosomes in translating mRNA, as well as ribosomal composition. Most recently, in 2020, high density, targeted monitoring tyrosine phosphorylation reveals activated signaling networks in human tumors.1
Mass spectrometry in the laboratory
Mass spec can identify the differences between cold, flu, COVID-19, and tuberculosis, and it shows the cells for global profiling. When used for single cell proteomics, heterogeneity, scarcity, novel cell types and cell state transitions are seen. In spatialomics, lab professionals can measure cell to cell variation, cellular and tissue heterogeneity and connectivity.1
Microbial identification has been advanced by mass spec, according to the American Chemical Society. Systems can identify more than 400 bacteria and yeast species for bacterial identification, with accuracy rivaling nucleic acid sequencing.3 Liquid chromatography with tandem mass spectrometry (LC-MS/MS), which combines the separating power of liquid chromatography with the mass analysis of triple quadruple mass spectrometry, advanced dried blood spot analysis and metabolism information, screening for more than 50 metabolic disorders with one rapid test, advancing newborn screenings.3
Through mass spectrometry, further analysis can be done with lipid chains, and the double bonds of steroids responding to UV for fragmenting small molecules. Closed cell protein can be researched, as well as protein and protein interactions of more complex biomolecules.
While immunoassays may overestimate steroids from cross-reactivity, LC-MS/MS has high accuracy for steroid analysis. Thus, thyroid assessment with isotope dilution will out perform equilibrium dialysis by being not only more accurate, but faster. Several biomarkers have been identified using LC-MS/MS, and while some may still be going through clinical trials, mass spec continues to advance analysis when combined with artificial intelligence to recognize patterns of biomarkers.3
Typically, in mass spec, analytes are introduced to a vacuum region prior to ionization; however, with ambient ionization mass spectrometry (AIMS), ions are formed outside of the mass spectrometer in atmospheric conditions and are pulled into the instrument for mass analysis. This is helpful in situ applications, being able to analyze intact subtances. Since desorption electrospray ionization (DESI), which combines desoption and ionization of anlytest from the surface of an untreated sample, and direct analysis in real time (DART), a plasma-based ambient ionization, were introduced, the field of ambient ionization sprouted with research opportunities. These techniques can probe live organs, skins, tissues and fluids open to the air to instantly capture molecular patterns,3 introducing such inventions as the MassSpec Pen to distinguish between normal and cancerous tissues.
The specs of mass spec
Direct Analysis in Real Time Mass Spectrometry (DART MS) is an ambient ionization technique on a gas-phase ionization mechanism, usually helium or nitrogen, initiating reactions, which can generate positive or negative ions, predominantly even-electron species, such as the technology used by IonSense. This can analyze analytes in their native form, including many that don’t ionize well in other methods, allowing it to be applied to compounds that have been deposited, or are being desorbed into the atmosphere, without the use of chemical solvents.
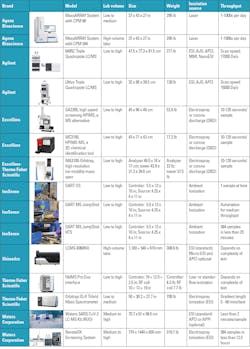
The ease of automated models for the labs can increase workflow efficiency, adding analytical intelligence to maximize laboratory outputs. Some models, such as Shimadzu’s LCMS-8060NX triple quadrupole liquid chromatograph mass spectrometer, uses ion focused lenses and integrates electrospray, so neutral particles are expelled to reduce noise and provide stability. Heat assists with desolvation, and ions are swept from the collision cell. On this model, the ESI spray needle and the inlet desolvation line (DL) can be changed without breaking the vacuum.
Excellims GA2200 is an alternative high speed screening high performance ion mobility spectrometer (HPIMS), which operates like a mass spectrometer using an electrospray ionization source that exceeds portable mass spectrometers. It can be used at the point of need, as it works without a vacuum pump and has no need for large quantities of solvents, with rapid results in 10-120 seconds.
Combining IMS and MS, the MC3100 HPIMS-MS is a 2D, portable chemical identification tool used for drug screening and more. Compact with benchtop performance, it integrates atmospheric pressure IMS with a linear ion trap MS, enabling chemical separation and identification based on ion mobility, then the same sample can be used in the MS for m/z identification in the field portable instrument.
Another compact design is the Agilent Ultivo liquid chromatography quadrupole mass spectrometer, which is stackable and smaller than the average triple quadrupole for liquid chromatography (LC/TQ) with the same power. Agilent’s VacShield offers ventless ion injector maintenance. This same VacShield, which reduces downtime with quick maintenance without breaking vacuum, is offered in Agilent’s larger 6495C Triple Quadrupole LC/MS with hexabore capillary and iFunnel Technology to reduce noise.
Excellims and Thermo Fisher Scientific combine forces with the MA3100 Orbitrap, an ion mobility pre-separator for mass spectrometers, which enhances versatility and more targeted analysis of gas phase ions, a process not always available by mass spectrometry alone. This is exchangeable with other ionization sources, providing add-on molecules to the ionization sources of the MS, enabling orthogonal separation, and it cleans up the ion beam for MS. So far, it is the only way to obtain “collision cross section” information for Thermo Fisher Scientific’s MS systems.
The Thermo Fisher Scientific Orbitrap IQ-X Tribrid mass spectrometer combines these technologies, and the Met-IQ workflow leverages Real-Time Library Search with MS spectral matching, with optional extras that include an Ultraviolet Photodissociation (UVPD) for lipid double-bond localization, site specific glucuronidation and fragmentation, as well as the 1,000,000 (1M) resolution option. The Thermo Fisher Scientific FAIMS Pro Duo interface extends differential ion mobility to proteomics, plasma profiling, and small-molecule quantification. Covering a wide range of chromatographic flow rates on different compound types, it overcomes matrix interferences by orthogonal selectivity leveraging gas-phase fractionation, enhancing signal-to-noise ratios. Integrating with liquid chromatography provides identification of proteins, peptides, oligonucleotides, and small molecules.
Waters RenataDX Screening System uses flow injection for high-throughput dried blood spot analysis. Accommodating samples in microtiter, deep-well plates or vials, both laboratory developed tests (LDT) and commercial reagent kits can be used. The RenataDX can receive barcode scanning information, and high-volume labs can add three extra trays to the standard 12-plate configuration. Expanding research opportunities, Waters SARS-CoV-2 LC-MS kit is for research use only (RUO) with an orthogonal analytical method to detect and quantify nucleocapsid peptides showing a lower limit quantitation of 3amol/µL.
The MassARRAY System by Agena Bioscience enables applications with off-the-shelf panels for pharmacogenetics, liquid biopsy, tumor profiling, sample identification, SARS-CoV-2 and variants, hereditary genetics, bone marrow engraftment chimerism, and blood typing. It supports rapid custom panel creation and can identify variant allele frequencies as low as 0.1%, while eliminating the need for fluorescent markets.
For genetic analysis, after extraction, nucleic acid is amplified and added to the MassARRAY for target sequence detection and data analysis. This process allows the identification of SNPs, insertions, deletions, translocations, copy number variation, and methylation markers. The reporting software delivers result reports without complex bioinformatics.
To help decipher results, ASCENT, from Indigo BioAutomation, is a software service that accelerates the release of high-quality both liquid and gas chromatography MS results. Although assays are built and validated on clean and well resolved peaks, sometimes the samples aren’t clean. Whether samples are near the baseline, suffer co-elution, or have lost sensitivity and specificity expected, ASCENT starts from an adaptive per-peak smoothing algorithm to minimize the amount of peak distortion, applying machine-learning techniques to distinguish the true peak signal within the chromatographic trace. The rules engine is customizable to the laboratory SOP, and there is high visibility into the audit trail, with cross-batch rules and cross-batch reporting built in.
With these new avenues of analysis, the possibilities for discovery are endless. It will be exciting to see what laboratorians research next, as ground-breaking technologies continue to develop in the field.
References
- Kennedy M. Thermo Fisher Scientific Chromatography and Mass Spectrometry 2021 Virtual Press Conference. Thermo Fisher Scientific. https://thermofisher-events.webex.com/recordingservice/sites/thermofisher-events/recording/90d7ddaf6a894a4d84c095d74507b7e2/playback. Accessed July 8, 2021.
- Griffiths J. A brief history of mass spectrometry. Analytical Chemistry. 2008;80(15):5678-5683. doi:10.1021/ac8013065
- Banerjee S. Empowering clinical diagnostics with mass spectrometry. ACS Omega. 2020;5(5):2041-2048. doi:10.1021/acsomega.9b03764
About the Author
Marisa L. Williams
Editor
The author of more than 100 independently published books, Marisa L. Williams earned her Master’s at Johns Hopkins University, while interning on Capitol Hill, doing press and communications for the National Association of Community Health Centers. Creating her own Bachelor of Science degree at the University of Toledo, Williams blended pre-medical, pre-law, and laboratory studies, resulting in an interdisciplinary degree emphasizing Forensics. She has worked with Dr. Paulette Moulton (Levy), a dermatologist in Monroe, MI, as well as Dr. Elizabeth Triana, a family medicine practitioner specializing in hormone therapy, based out of Port Charlotte, FL; additionally, she has 20 years of experience as a multimedia journalist, is a third generation Realtor licensed in MI and FL, and has five years of college level teaching experience.