Every needlestick is one too many: Progress made and still to come for sharps injury prevention
Needlestick injuries are not new to the world of healthcare. In the United States, awareness around prevention began gaining momentum in the early 1990s — more than 30 years ago — with the rise of the human immunodeficiency virus (HIV) and hepatitis B and C epidemics. The Occupational Safety and Health Administration (OSHA) published the Occupational Exposure to Bloodborne Pathogens Standard in 1991 to help protect healthcare workers from the risks of needlestick injuries and subsequent exposure to bloodborne pathogens.
In 2000, the Needlestick Safety and Prevention Act (NSPA) was signed into law, effectively amending OSHA’s Bloodborne Pathogens Standard to more specifically define the types of engineering controls and devices required to improve safety, require employers to maintain a sharps injury log, and require employers to consider the input of frontline staff in the evaluation and selection of safety-engineered devices.
Both of those early milestones were significant in highlighting the risk of needlestick injuries and establishing best practices for prevention, and data shows progress toward reducing the instances of needlesticks. However, data also shows that progress has slowed or even reversed in recent years.1 Today, the Centers for Disease Control and Prevention (CDC) estimate there are approximately 385,000 needlesticks and other sharps-related injuries to hospital-based healthcare personnel each year.2
Renewing safety focus amidst a rapidly changing healthcare landscape
Most sharps injuries involve physicians and nurses, but anyone who encounters needles in the course of their work is at risk. This includes roles from laboratory staff, nurse assistants, and medical technicians to environmental services. With a workforce already stressed by understaffing and turnover — exasperated by the COVID-19 pandemic — it is critical to consider that workers with varying levels of experience and training handling sharps may encounter them. That is why OSHA’s Bloodborne Pathogens standard stresses the creation of exposure prevention plans and regular training.
Five ways to prevent sharps and needlestick injuries, according to OSHA:3
1. Plan safe handling and disposal of sharps before any procedure.
2. Use safe and effective needle alternatives when available.
3. Activate the device’s safety features immediately after use.
4. Immediately dispose of contaminated needles in OSHA-compliant sharps containers.
5. Complete bloodborne pathogens training.
These steps may seem simple, but they are also easy to take for granted. For instance, device disposal into an appropriate sharps container. While 50.4% of sharps injuries in 2023 occurred during device use, before any safety mechanisms were activated, it is also worth noting that approximately 3% of sharps injuries occurred during disposal — the majority of which came from devices protruding from sharps containers.1
That was the case for Karen Daley, PhD, RN, MPH, FAAN. Dr. Daley, a past president of the American Nurses Association, spent more than 25 years in clinical practice at Brigham and Women’s Hospital in Boston until July 1998, when she was stuck by a needle protruding from a sharps container while disposing of a device. Needlesticks were not uncommon, and she did not think much of the incident at first.
“I never expected I would get infected with HIV and hepatitis C from a needlestick injury at work,” said Dr. Daley. “I almost did not report my exposure, but I’m grateful I was encouraged to. If I could change the circumstances contributing to my injury and infection, there are things I would have done differently. I would have made sure the needle disposal box was not overfull. I would have agreed to take the recommended post-exposure prophylaxis. All healthcare workers need to take advantage of the tools and information available to keep ourselves safe.”
Dr. Daley shares her story now to raise awareness of the risks surrounding needlestick injuries. Since her departure from clinical practice, she has been actively engaged on state, national, and international levels as an advocate for the use of safer needle devices in healthcare practice settings. She was present when then-President Bill Clinton signed the NSPA into law in 2000.
“Seeing the power of coalition building, telling my story, and being part of the effort to get safety reform legislation passed have all been incredibly rewarding parts of my experience,” she said.
Another example of injury during device disposal comes from Annette Estrada, Greiner Bio-One Product Specialist for Preanalytics.
“In my 45 years of working in the clinical lab, I witnessed several coworkers sustain needlestick injuries,” Estrada said. “When it happened to me, I could not believe it — until I saw the punctured glove and blood.”
“My injury occurred with a butterfly device that did not have a safety mechanism. Upon completing the venipuncture, when trying to discard the device, one of the wings caught the edge of the sharps disposal box opening. The device slipped, sticking the palm of my hand.”
“I can’t help but think,” she said, “’If that butterfly had a safety device, I would have never sustained that needlestick.”
Stories like those from Dr. Daley and Estrada highlight a simple truth: Every needlestick is one too many. Even in best-case scenarios where there is no subsequent infection, the stress of an injury, risk of exposure to bloodborne pathogens and rigor of follow-up care place an incredible burden on healthcare workers who sustain needlesticks.
Engineering the future of needlestick injury prevention
When considering what measures may help prevent future needlesticks, engineering controls are a key focus. Data shows that needles and sharp devices without safety mechanisms accounted for 66.9% of sharps injuries in 2023.4 The CDC estimates that 62 to 88 percent of sharps injuries can be prevented simply by using safer medical devices.5 The Clinical & Laboratory Standards Institute (CLSI) standard for collecting venous blood specimens strongly encourages the use of safety-engineered blood collection devices.6 OSHA’s Bloodborne Pathogens standard requires their consideration, stating: "Engineering and work practice controls shall be used to eliminate or minimize employee exposure.”7 This means that if an effective and clinically appropriate safety-engineered sharp exists, an employer must evaluate and implement it.
The decades since OSHA’s original standard publication in 1991 and NSPA’s passing in 2001 have been marked by innovation in the design of safer sharps devices. Clinical laboratory practitioners see the fruits of this effort in improved blood collection devices, ranging from basic, manually assembled and activated needle shields to more advanced pre-assembled and semi-automatic devices.
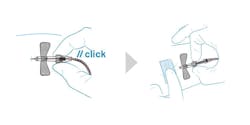
Devices that enable in-vein activation – meaning the needle is completely and irreversibly sheathed as it is retracted from the patient’s vein, as illustrated in Figure 1 – offer some of the most advanced safety mechanisms among blood collection needles.
Taking responsibility for needlestick safety and prevention
Even with safety-engineered devices, proper training and education are essential to successfully reducing the rate of needlestick injuries. According to EPINet data, 27.3% of sharps injuries in 2023 were sustained while using safety-engineered devices.4 One study conducted in the Netherlands in 2018 even suggested the incidence of needlestick injuries may have increased since the wide adoption of safety- engineered devices. That study went on to identify two primary causes that may be improved with additional training: difficulty operating the devices and continued improper disposal.8
The Bloodborne Pathogens standard explicitly requires employers to provide bloodborne pathogen training to employees at the time of initial assignment and at least annually thereafter. CLSI GP41 stipulates using all safety-engineered devices according to the manufacturer’s instructions for use. Both standards call for immediate, safe disposal of used devices into appropriate sharps disposal containers.
As Dr. Daley suggested, protecting healthcare workers from needlesticks and sharps injuries requires a coalition approach. Manufacturers are responsible for continually improving and enhancing devices for safe, simple use; facility leadership is responsible for providing safe work environments, training, and tools to help protect healthcare workers; the device users themselves are responsible for taking ownership of their personal safety and following best practices to protect themselves and others.
Conclusion
Significant progress has been made in the past three decades as a result of combined focus and attention on needlestick safety and prevention. However, recent data shows the work is far from completed. As a recent consensus statement from the International Safety Center says, “Healthcare workers represent a critical national resource that must be protected from harm while they care for others. Healthcare worker safety is a crucial component of patient safety, and of the overall safety and quality of the healthcare environment.”1 Because every needlestick is one too many, progress must continue to ensure safer devices and safe practice.
References
1. 2020 Consensus Statement, Call to Action. Moving the sharps safety in healthcare agenda forward in the United States: Internationalsafetycenter.org. Accessed July 25, 2024. https://internationalsafetycenter.org/wp-content/uploads/2020/12/Moving_The_Sharps_Safety_In_Healthcare_Agenda_Forward_In_The_US.pdf.
2. Centers for Disease Control and Prevention. Sharps safety program resources. Infection Control. Published May 15, 2024. Accessed July 25, 2024. https://www.cdc.gov/infection-control/hcp/sharps-safety/index.html.
3. Occupational Safety and Health Administration. Bloodborne Pathogens and Needlestick Prevention. Occupational Safety and Health Administration, United States Department of Labor. Accessed July 25, 2024. www.osha.gov/bloodborne-pathogens.
4. Jan RP, Dec 2023 to, Read: TR. EPINet Report for Needlestick and Sharp Object Injuries | 2023. Internationalsafetycenter.org. Accessed July 26, 2024. https://internationalsafetycenter.org/wp-content/uploads/2024/05/Official-2023-US-NeedleSummary-1.pdf.
5. Occupational Safety and Health Administration. "Evaluating and Controlling Occupational Exposures to Bloodborne Pathogens." Occupational Safety and Health Administration, United States Department of Labor. Accessed July 26, 2024 www.osha.gov/bloodborne-pathogens/evaluating-controlling-exposure.
6. Clinical and Laboratory Standards Institute. Collection of Diagnostic Venous Blood Specimens. 7th ed. CLSI standard GP41. Wayne, PA: Clinical and Laboratory Standards Institute; 2017.
7. Occupational Safety and Health Administration. "Occupational Exposure to Bloodborne Pathogens (29 CFR 1910.1030)." Occupational Safety and Health Administration, United States Department of Labor. Accessed July 26, 2024. www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030.
8. King KC, Strony R. Needlestick. StatPearls Publishing; 2023.