Navigating the medical lab landscape: Key trends in costs, contracting, staffing, and technology
As U.S. medical laboratories adapt to evolving challenges, both ongoing (e.g., staffing issues and cost pressures) and emerging, respondents to the 2025 MLO State of the Industry (SOI) survey on lab management best practices remain steadfast in some priorities and practices while shifting focus in others.
This year, MLO gathered responses from 180 clinical laboratory professionals, with 43% of respondents in director, manager, administrator, or supervisor positions, and most employed by hospitals (59%). Although respondents spanned labs of different sizes and testing volumes, there was a notable increase in those in labs performing more than 2,000,000 tests annually (20% in 2025, up from 12% in 2024).
Alongside the quantitative survey results, MLO presents commentary from medical laboratory professionals and technology solutions providers.
Six key survey findings
- Costs and reimbursements: Standardized instrumentation workflows, checklists, lab processes, staff education, and IT solutions to reduce human error were cited as top best practices in ensuring reimbursement covers lab costs. Adoption of processes to review savings opportunities (e.g., regular analyzer evaluations) dropped in best practices rankings.
- Contracting: More labs are working with their supply chain management teams to identify group purchasing organization (GPO) contract savings opportunities, with a marked increase over last year. Fewer view relationship building with supplier support personnel and associated opportunities for training and product optimization suggestions as a best practice for streamlining contracting processes.
- Supply chain: More than one-third (32%) of survey respondents report they are not experiencing supply chain issues, which begs the question, “Are the remaining 68% still facing issues?”
- Staffing and labor: Financial incentives, continuing education offerings and partnerships with local colleges and tech schools were cited as key best practices for recruiting and retaining lab staff. Several professionals commented on international recruiting. Less prevalent this year was reported shift changes to offer employee scheduling flexibility.
- Technology priorities: Labs prioritize technology needed to cover broken/older equipment or improve quality/reduce costs in their capital budget. Fewer give precedence to technology needed to cover staff shortages with automated equipment this year compared with last.
- Laboratory-developed tests (LDTs): More than half of lab professionals surveyed do not have LTDs in their labs. Among those with LDTs, nearly one-third were still evaluating how to comply with Stage 1 of the U.S. Food and Drug Administration’s (FDA) final rule.
Reimbursement, revenue, and contracting trends
To ensure reimbursement covers their costs, more than half of laboratory professionals surveyed have standardized instrumentation workflows and checklists (53%, down from 58% in 2024) or created standard lab processes and staff education materials (52%, down from 56% in 2024). Close to half have incorporated IT solutions to reduce human error (46%, down from 50% in 2024).
More than one-third have adopted the use of analyzers that provide walkaway testing to reduce staffing and full-time equivalent (FTE) (37%, down from 41% in 2024) and nearly one-third report incorporating IT solutions to help keep current with regulations (29%, down from 37% in 2024).
Close to one-quarter of respondents have adopted process to review savings opportunities, such as evaluating analyzers on a regular schedule (23%, down from 36% in 2024), or implemented ongoing waste and efficiency studies to find potential savings in overhead (23%, down from 24% in 2024).
Others report having brought health screening tests in house (17%, down from 26% in 2024) or have implemented ongoing efforts to reduce coding frustrations and modifications (17%, down from 21% in 2024).
Additionally, 16% of lab professionals indicated they have implemented other steps to ensure reimbursement covers their costs. In their comments, some manually perform this work, including staff audits of service fees, reimbursement, reasonable cost and nursing billing for transfusions and point of care testing (POCT) performed.
Others noted how this work is done at a corporate level or by another department outside of the lab. One survey respondent wrote how their lab evaluates whether reimbursement pricing for participating insurance will cover costs before implementing new tests.
For those labs that cannot track whether reimbursement covers their costs, common stumbling blocks include:
- 34%: Not having software to automate tracking/analysis of costs (up from 27% in 2024)
- 29%: Lack of interoperability between laboratory information system (LIS) and revenue cycle management software (up from 24% in 2024)
- 26%: Not having enough lab staff time (down from 34% in 2024)
- 23%: Not having enough IT staff time/resources (new answer option for this year)
- 4%: Not having enough barcoded testing supplies (down from 6% in 2024)
Juan Munoz, Director of Technical Solutions, Electronic Imaging Materials, commented on barcode trends and considerations, stating:
“Barcode labeling in the laboratory is indispensable and there are many factors to contemplate. The overall application and environmental conditions will have an impact on what type of label should be utilized (matte/gloss finish, adhesive type, etc.).”
Munoz went on to present the factors that influence barcode symbology and size, including the “amount of data, the printable real estate available, and the scanning technology being used.”
“If printing in-house, the size of the barcode and the symbology to be printed will determine what the printer’s resolution will need to be,” Munoz explained. “And as mentioned, the scanning technology must be compatible with the barcode symbology.”
An additional 15% of lab professionals surveyed cited other reasons for why they cannot track whether reimbursement covers their costs, including lack of expertise in report writing for near-real time tracking, delays in being reimbursed to reconcile records, and the inability to easily differentiate which diagnostic-related group (DRG) costs should be assigned to the lab.
One respondent noted how management performs this tracking and does not share information with the team unless needed. Another wrote how their team looks at reimbursement coverage when they are offering a new test/method and/or an auditor alerts them to a potential major change in reimbursement.
When it comes to best practices in streamlining the contracting process, 64% have worked with supply chain management to identify supplies on group purchasing organization (GPO) contracts that offer additional savings (up from 55% in 2024).
Among write-in comments, one lab professional explained how their supply chain team is bringing in suppliers with less expensive products for the lab team to evaluate. Another wrote how they are working with supply chain on contract review and optimization to renegotiate existing contracts based on current spend.
Other best practices in the survey included the development of good relationships with supplier support personnel to secure access to training and product optimization suggestions (44%, down from 61% in 2024), and adoption of ongoing reviews of reference lab costs and contracts (41%, up from 39% in 2024).
Additionally, 21% of survey respondents indicated they have signed longer contracts (for example, 7 years instead of 1–3 years) up from 16% in 2024, while one lab professional in their write-in comments noted how their lab is securing shorter contact terms due to declining payments terms.
Staffing and labor trends
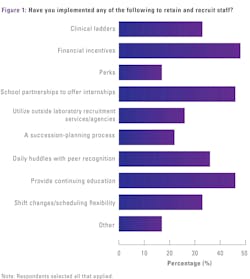
Given ongoing shortages of qualified medical laboratory personnel, MLO asked its readership what practices they have implemented and benefits they have offered to retain and recruit staff.
Topping the list were:
- 48%: Financial incentives, such as sign-on bonuses, merit allowances and retention bonuses (up from 42% in 2024)
- 46%: Continuing education offerings (down from 50% in 2024)
- 46%: Partnerships with local colleges and tech schools to offer internships in their labs (up from 38% in 2024)
Next in the top-ranking practices and benefits employed were:
- 36%: Daily huddles with peer recognition (unchanged from 2024)
- 33%: Clinical ladders/structures to encourage professional development, such as from novice to expert (down from 40% in 2024)
- 33%: Shift changes to offer employee scheduling flexibility (down from 44% in 2024)
Further down on the list were:
- 26%: Utilizing outside laboratory recruitment services/agencies (new answer option for this year)
- 22%: Succession-planning processes by offering additional responsibilities to top performances and measuring results (up from 19% in 2024)
- 17%: Perks, such as free parking, onsite gym, onsite day care, reimbursed public transportation costs (unchanged since 2024)
Among lab professionals indicating the application of other practices and benefits to retain and recruit staff (17%), several wrote they are recruiting and hiring internationally by leveraging H1B visa sponsorship. One survey respondent wrote how their lab offers an Employee Assistance Program (EAP), while another offers employees health services, such as free flu and HBV vaccines.
“While automation has helped in the lab, it hasn’t eliminated the need for qualified talent,” said Liz Nesladek, Chief Commercial Officer (CCO), Conexus MedStaff. “Many labs find that talent planning with international staff can fill the talent gap. Some might argue that the visa process takes too long, but the reality is most cases don’t take any longer than hiring and retaining domestic staff.”
“As the immigration sponsor, Conexus takes the burden off our lab clients, finding and hiring the right qualified MLS for their needs, and we continue to provide support for the duration of each international MLS’ 3-year employment term, including immigration, training, and credentialing,” she added.
Supply chain trends
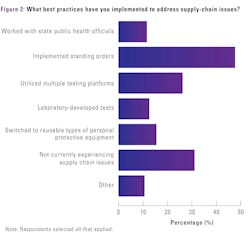
More than one-third (32%) of survey respondents report they are not experiencing supply chain issues. Compared with the 2024 survey results, more lab professionals are implementing standing orders (instead of just in time) for crucial supplies (49%, up from 37% in 2024).
There were slight dips in respondents who report working with state public health officials to gain access to needed testing supplies (12%, down from 16% in 2024), utilizing multiple testing platforms (27%, down from 30% in 2024), and switching from disposable to reusable types of personal protective equipment (16%, down from 20% in 2024).
There was no change year-over-year in lab professionals who report using LDTs to address supply chain issues (13%).
When asked what steps they have taken to improve inventory control and consumable supply costs, evaluation of inventory levels for basic supplies, such as assays and controls/reagents, topped the list (64% down from 69% in 2024). The second highest percentage of responses was for the development of supply utilization tracking and record keeping (38%, down from 46% in 2024).
This was followed by:
- 18%: Secured access to electronic inventory tracking from the supply chain/materials management department (down from 20% in 2024)
- 17%: Worked with other members of the organization, such as the Chief Medical Officer (CMO) and physicians, to standardize test ordering throughout the organization (down from 24% in 2024)
- 16%: Developed ongoing review comparing supply reports to the number of invoiced tests (down from 19% in 2024)
- 15%: Implemented vendor-managed ordering (up from 12% in 2024)
- 11%: Implemented lease agreements that do not include volume commitments (down from 14% in 2024)
- 9%: N/A this is handled by a different organization/location (down from 12% in 2024)
Testing trends
Since the FDA published its Final Rule on LDTs on April 29, 2024, medical laboratory professionals and other industry stakeholders have raised questions around the impact of the regulation. A month after readers responded to this survey, the U.S. District Court for the Eastern District of Texas granted AMP’s motion for summary judgment and vacated the FDA rule that would have regulated LDTs.
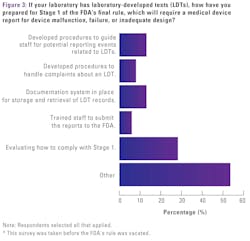
MLO had asked survey participants how they prepared for Stage 1 of the FDA’s final rule, which would have required a medical device report for device malfunction, failure or inadequate design. Over half of respondents (53%) indicated they do not have LDTs in their labs.
For those with LDTs:
- 28% are still evaluating how to comply with Stage 1
- 13% have developed procedures to guide staff for potential reporting events related to LDTs
- 13% have a documentation system in place for storage and retrieval of LDT records
- 8% have developed procedures to handle complaints about an LDT
- 6% have trained staff to submit the reports to the FDA
Comments provided by respondents included:
- “We improve all testing for better results even though it may differ from the manufacturer's instructions.”
- “We are developing a plan to comply but there remain many unanswered questions.”
- “[We have] incorporated [LDT compliance] into nonconformance and risk management.”
- “[We’ve had] LDTs for several years now, submitted for approval to regulatory agencies, and conduct and perform proficiency testing.”
- “We have identified our procedures and are in the process of implementation.”
When it comes to steps labs are taking to improve the quality and efficiency of their overall testing, half of those surveyed have staff or committee members review their standard operating procedures (SOP) for testing (50%, up from 48% in 2024), and nearly half have implemented standardized test ordering procedures and formularies (48%, down from 52% in 2024).
“Quality control is not just a requirement - it is the foundation of laboratory excellence,” said Dr. Stephen Pelsue, Manager of Discovery, Maine Molecular Quality Controls (MMQCI). He explained:
“In molecular diagnostic laboratories, quality controls ensure the accuracy and reliability of laboratory results necessary for high quality patient care. Best practices include validated reagents, routine instrument calibration, and standardized protocols and regular proficiency testing, internal and external controls, and contamination prevention to safeguard results. By integrating these measures, labs minimize errors, improve patient outcomes, and uphold trust in diagnostics.
“Troubleshooting and recovery after an out-of-control condition have always been difficult topics for labs to address,” said John C. Yundt-Pacheco, Senior, Principal Scientist/Scientific Fellow, Bio-Rad Laboratories Quality Systems. “This year, we anticipate emerging industry trends may help with these issues.” Elaborating on these trends, he stated:
“I think we will see increased adoption of best practices such as estimating how many patient samples are likely to be outside quality specifications given the magnitude of the error condition; identifying the point of failure; suggesting a recovery approach - either spot checking a critical list of samples or back-testing; guidance on how much back-testing is required; and which samples need correction.”
Following close behind are those who have evaluated temperature monitoring equipment and procedures (42%, up from 39% in 2024). Further down on the list, 20% have implemented a pre-approval program for send-out tests (up from 14% in 2024), and 19% have implemented evidence-based test utilization backed by data (down from 28% in 2024).
Ray Almgren, CEO, Swift Sensors, commented on trends in temperature monitoring, stating:
“Historically, temperature monitoring was accomplished by lab techs physically reading thermometers throughout the lab and recording temperatures in a written log on a schedule. Over the years, wired sensors tied to internal networks automated some of the manual tasks and provided some data management capabilities.”
“Modern temperature compliance solutions combine wireless temperature sensor technology, advanced networking protocols, AI and cloud-based software platforms to improve usability, convenience, accuracy and reliability,” he added. “It’s the right time to upgrade due to these technology advancements.”
Technology trends
Turning to best practices lab professionals have implemented for adopting new tools for laboratory automation:
- 44% have analyzed workflow processes for proper space planning (up from 43% in 2024)
- 40% have involved their IT department early in the process (up from 37% in 2024)
- 36% have ensured system integration for seamless process and data flows (up from 33% in 2024)
- 21% have designated a project manager to coordinate short- and long-term planning and implementation with the vendor (unchanged since 2024)
Ed Nesbitt, Vice President of Service, CompuGroup Medical, commented on how laboratory information system (LIS) interoperability has “significantly evolved since the early days of lab management,” stating:
“In the past, a laboratory may have relied on a single, standing Health Level 7 (HL7) interface to facilitate communication with other healthcare systems. Today, laboratories navigate a complex web of interoperability demands including multiple data exchanges with various electronic health records, billing systems, and public health agencies.”
“A successful implementation requires expert planning,” he continued. “Each implementation should be tailored to meet unique customer needs and specific vendor requirements. The goal is to create an optimal, patient-centered outcome with a foundation around data accessibility and accuracy.”
Among best practices labs have developed to train staff on new software:
- 55% have created standard workflows for all lab employees (up from 52% in 2024)
- 48% have created a train-the-trainer model (down from 54% in 2024)
- 37% have employed vendor-hosted in-house or online training (up from 30% in 2024)
- 32% have had a lab person receive LIS training to develop an in-house expert (unchanged since 2024)
- 24% have developed mandatory training for new lab employees that is led by the IT department (unchanged since 2024)
- 19% have developed lunch-and-learn training sessions (down from 22% in 2024)
Among lab professionals providing write in comments, one noted how basic training is given by fellow lab employees and another wrote how their lab has created detailed SOPs for lab techs to follow, along with practice cases to learn new workflows.
When asked how their labs prioritize technology needs for their capital budgets, 59% prioritize technology needed to cover broken/older equipment (down from 60% in 2024) and 57% prioritize technology needed to improve quality/reduce costs (down from 62% in 2024).
Slightly more respondents report prioritizing technology needed to remain competitive (38%, up from 35% in 2024), while there was a modest drop in those prioritizing technology needed to cover staff shortages with automated equipment (29%, down from 40% in 2024).
Other priorities cited include:
- Technology to standardize processes
- Technology to better reimbursement and reduced costs
- Technology needed to improve quality of patient care
- Cost per test contracts and duplicate analyzers to provide state-of-the-art testing capabilities
Conclusion
The findings from the 2025 MLO State of the Industry survey on lab management best practices highlight both persistent and shifting priorities among medical laboratories as they navigate financial constraints, staffing shortages and regulatory changes.
As the industry moves forward, laboratories will need to remain agile in their approach to workforce management, financial sustainability, and compliance with evolving regulations.
About the Author

Kara Nadeau
has 20+ years of experience as a healthcare/medical/technology writer, having served medical device and pharmaceutical manufacturers, healthcare facilities, software and service providers, non-profit organizations and industry associations.