Because it is useful in the diagnosis and monitoring of renal and urinary tract diseases, urinalysis is one of the most commonly ordered laboratory tests. Basic urinalysis includes macroscopic examination, chemical analysis, and microscopic sediment examination. Although associated with significant labor and training requirements, manual microscopy remains the gold-standard methodology for sediment analysis; however, automated instruments are a valuable tool in the clinical lab.
Numerous studies have been performed comparing the performance of automated instruments to manual microscopy. Herein we provide a brief overview of the available technologies and conclusions from some of the recently published studies.
Overview of technologies
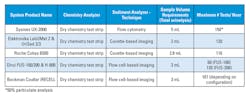
There are two types of technologies available for urine sediment analysis: flow cytometry and digital imaging techniques. These methods are summarized below, with additional technical specifications included in Table 1. A brief mention of the optional chemistry analyzers is also included for each sediment analyzer.
Flow cytometry can be used to identify and quantify cells, casts, bacteria, and other particles in urine sediment. Addition of fluorescent stain that binds to microbes in the specimen adds sensitivity and specificity for detection of smaller pathological elements. As particles pass through a flow cell, they are illuminated by a laser. The elements in the flow cell are classified according to impedance, light scatter, and fluorescence. Results are viewed as scattergrams with the numeric counts of each sedimentary element appearing as a distinct cluster. This method has been used for detection and quantitation of erythrocytes, leukocytes, hyaline casts, bacteria, and epithelial cells. Other elements such as crystals, yeast, oval fat bodies, sperm, mucus, and pathological casts are also detected but are not readily quantified. Specimens containing these elements are flagged for review and quantitation by manual microscopy.
Currently, Sysmex is the only company offering a flow cytometry-based instrument for clinical urinalysis. This family of analyzers consists of several instruments, the newest stand-alone sediment analyzer being the UF-1000i. A combined platform performing automated chemical and sediment analysis was recently released as the UX-2000.1-3
As for digital imagining techniques, identification of pathologic elements in urine sediment can also be accomplished using automated imaging. In this approach, a collection of high-resolution digital images are captured and then analyzed by image and pattern recognition software. There are two basic designs for collection of digital images in automated instruments; cuvette-based and flow cell systems.
In cuvette-based platforms, a urine sample is briefly and gently centrifuged in a specially-designed cuvette, resulting in a monolayer of particles. An automated, bright field microscope (typically with 400x magnification) then captures 10 to 20 images of the deposited particles on the surface of the cuvette. Images of the particles are displayed as whole-field views, similar to manual microscopy, allowing reviewers to visualize and manually verify the presence of pathological elements. Image processing software automates the process of identifying and categorizing particles with the help of a comparative reference image library.
The Hungarian company 77 Elektronika introduced UriSed (SediMAX in some market regions) in 2009. Since then, it has released UriSed 2, and more recently, UriSed 3. This latest iteration incorporates phase contrast microscopy in addition to bright field, to improve differentiation of elements such as hyaline casts, red cell membranes, crystals, and yeast. Images are evaluated in real-time using the company’s Auto Image Evaluation Module (AIEM). UriSed 2 or UriSed 3 can be linked with the chemistry analyzer LabUMat 2 (or AutioMAX to SediMax) for full automation of urine chemistry and sediment analysis.1,3,4
Roche Diagnostics has entered the market for automated urine sediment analysis with the cuvette-based Cobas u701. However, the instrument is not yet available in the U.S. It provides quantitation of erythrocytes and leukocytes, semi-quantitative assessment of bacteria, epithelial cells, and hyaline casts, and qualitative evaluation of pathologic casts, crystals, yeast, sperm, and mucus. For complete automated urinalysis, the Cobas 6500 couples the u701 module with a u601 urine chemical analyzer.2,5
Flow cell digital imaging
Flow cell digital imaging techniques are also in clinical use. Flow cell analysis of urine sediment captures images in dynamic fluid rather than of a static surface as in the cuvette-based method. After images are collected, particles are identified and quantitated using image recognition software and comparison libraries. Urine is aspirated into the instrument and laminar flow is used to hydrodynamically orient particles as they pass through a flow cell. Digital images are captured and particles are classified based upon morphological features.
Beckman Coulter offers the Iris iQ200 family of instruments based on its proprietary Digital Flow Morphology for controlling flow characteristics and Auto-Particle Recognition (APR) software for identification and characterization of elements in urine. The camera captures ~500 frames per sample, and the instrument provides an interface for on-screen verification and review of results. The instrument is capable of differentiating erythrocytes, leukocytes, hyaline casts, unclassified casts, epithelial cells, bacteria, yeast, crystals, mucus, sperm, and amorphous substances. There are several iQ200 platforms available, iQ200SELECT, iQ200ELITE, and iQ200SPRINT, that can be linked with iChemVELOCITY to create the iRECELL platforms for total urine analysis.5,6
The Chinese laboratory diagnostics company DIRUI has been rapidly expanding its line of urinalysis instruments. The FUS-100 and FUS-200 urine sediment analyzers are also based on flow cell imaging technologies. The instruments use Flat Flow Digital Imaging technology, a trained neural network, and Artificial Imaging Identification (AII). A digital camera captures up to 820 images per sample (depending on the model) and AII identifies and classifies particles based on shape, contrast, texture, and frequency domain features. The FUS instruments can be combined with the H-800 chemistry analyzer for total urinalysis.4,6
Comparative instrument performance
Selecting the “best” design for urine sediment analysis is a complicated endeavor. Due to inherent differences in detection methodology, direct comparison between automated platforms is not straightforward. To overcome this challenge, element counts from an automated platform are generally compared to matched results from manual microscopy. Analytical performance characteristics for each class of particulate can then be reported. Sensitivity and specificity have been commonly used, as well as negative and positive predictive value. The agreement criteria often vary by element, and can be based upon presence vs. absence (e.g., patholgic casts) or agreement within a semiquantitative grade (e.g., RBCs of greater than 3, 4-10, etc.) Some studies instead report a more qualitatively derived concordance between methods, with an accompanying statistic such as Cohen’s kappa or the intraclass correlation coefficient (ICC). When comparing results from different studies, it is also important to consider that individual labs establish cutoffs based on their patient population as suggested by CLSI guidelines.
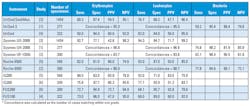
Table 2 summarizes select studies between 2013-2017 reporting analytical characteristics for clinical automated urine sediment analyzers. The majority of reports focus on the accuracy of automated detection and quantitation of erythrocytes and/or leukocytes relative to manual microscopy. Performance characteristics from these reports show the analytical capabilities of these instruments.1-6 Analytical sensitivity and specificity for erythrocyte and leukocyte detection was reported in four of six studies. The remaining two studies use concordance statistics in their analyses. Only two studies report statistics for detection of bacteria in urine sediment.
In Table 2, the analytical sensitivity and specificity for any given instrument can be widely variable when compared to manual methods. However, some generalizations can be made. In practical terms, identification rates of pathological features are clinically similar between flow cytometry and image-based methods. While images have the advantage that they allow operator review verification, this step does not appear to provide a substantial gain with regard to identifying erythrocites and leukocytes. Flow cytometry may have a slight advantage in recognition of bacteria due to the inclusion of bacteria-specific dye in reagents. In general, all automated instruments struggle with discrimination of crystals, yeast, pathological casts, and other pathological elements. Thus, algorithms are required to identify samples that need manual microscopy to detect them.
In summary, automated urine sediment analysis technologies continue to improve. As indicated by the relatively high specificity in most studies, automated analyzers are very useful for ruling out the presence of pathologic particles in urine. However, there remains a need for manual microscopy performed by experienced laboratorians to confirm abnormal findings. This is the practice that is followed at our institution, and in our patient population manual microscopy confirmation is required in 25 percent to 30 percent of samples, whereas the remainder can be reported based upon automated analyses.
REFERENCES
- Laiwejpithaya S, Wongkrajang P, Reesukumal K, et al. UriSed 3 and UX-2000 automated urine sediment analyzers vs manual microscopic method: A comparative
performance analysis. J Clin Lab Anal. 2017. - Lee W, Ha JS, Ryoo NH. Comparison of the Automated cobas u 701 Urine Microscopy and UF-1000i Flow Cytometry Systems and Manual Microscopy in the Examination of Urine Sediments. J Clin Lab Anal. 2016;30(5):663-671.
- Sanchez-Mora C, Acevedo D, Porres MA, et al. Comparison of automated devices UX-2000 and SediMAX/AutionMax for urine samples screening: A multicenter Spanish study. Clin Biochem. 2017;50(12):714-718.
- Yuksel H, Kilic E, Ekinci A, Evliyaoglu O. Comparison of fully automated urine sediment analyzers H800-FUS100 and LabUMat-UriSed with manual microscopy. J Clin Lab Anal. 2013;27(4):312-316.
- Bakan E, Ozturk N, Baygutalp NK, et al. Comparison of Cobas 6500 and Iris IQ200 fully-automated urine analyzers to manual urine microscopy. Biochem Med (Zagreb). 2016;26(3):365-375.
- Ince FD, Ellidag HY, Koseoglu M, et al. The comparison of automated urine analyzers with manual microscopic examination for urinalysis automated urine analyzers and manual urinalysis. Pract Lab Med. 2016;5:14-20.
Stacy M. Kenyon, PhD, is a secondyear clinical chemistry fellow in the Department of Laboratory Medicine and Pathology at the Mayo Clinic in Rochester, MN.
Kendall W. Cradic, PhD, is a fellow in Clinical Chemistry at the Mayo Clinic in Rochester MN.