In the history of modern medicine, few screening programs have been able to boast the same level of success in disease prevention as the simple Pap (Papanicolaou) test. What makes this achievement especially remarkable is that the cervical cancer screening protocol was established without a clear understanding of what caused the disease.
As the causal role of HPV, and in particular HPV genotypes 16 and 18, in the development of cervical cancer has become clearly established, it has naturally led to studies to identify what role HPV testing could play in screening programs. Findings to date have triggered landmark changes in screening guidelines. But recent data is pointing to the potential for an even more prominent role for HPV testing in screening programs around the world.
Identifying the Pap gap
In the history of Pap cytology-based screening in developed countries, one of the persistent dilemmas has been how to manage the relatively large number of colposcopies required to detect clinically important disease. A couple of decades ago, HPV testing was introduced as a triage test for the most nebulous of Pap test results: the ASC-US (Atypical Squamous Cells of Undetermined Significance) classification.
Over the years, a substantial body of evidence has developed revealing specific limitations of cytology-based screening, including the following:1-3
- Low reproducibility for ASC-US and LSIL (low grade squamous intraepithelial lesion)
- Lack of specificity for precancerous lesions in ASC-US
- Highly variable results among laboratories
- Poor performance in detecting adenocarcinoma
- Frequent screening requirement (minimum every 2 to 3 years)
One of the most revealing findings was that, despite the global impact of Pap testing in reducing cervical cancer mortality, up to 32% of cervical cancer occurs in appropriately screened women with normal Pap cytology results.4,5
It has become clear that normal cytology does not always mean cancer-free and that there are multiple limitations to using cytology alone in screening protocols. In addition, there has been growing concern that the frequency required for Pap tests to be effective in cervical cancer screening leads to a large number of false positives and unnecessary colposcopy. This has the potential to harm otherwise healthy women. Clearly there was an opportunity to improve on existing screening paradigms.
The rise of HPV genotyping
Concurrently, other clinical trials began to highlight the valuable role HPV genotype testing can play in assessing cervical cancer risk.
Several studies comparing HPV DNA testing and cytology showed that HPV testing was significantly more sensitive for the detection of precancerous lesions.6 A randomized screening trial in Italy demonstrated that HPV testing allows detection and treatment of precancers missed by cytology, thereby preventing more cancer than cytology alone.7 A Dutch screening trial also showed that HPV testing adds value over cytology testing in that the incidence of cervical cancer could be reduced when HPV testing was used.8
Further, baseline data from the U.S.-based ATHENA trial, which involved more than 47,000 women and began publishing results in 2011, demonstrated that one in 10 women positive for HPV 16 and/or 18 had high-grade cervical disease that was missed by cytology at baseline.9 The study established the utility of an HPV genotyping test to help stratify patient risk when used as a screening co-test with cytology for women age 30-plus9 or as a triage test for ASC-US cytology results in women 21 and older.10
Another key factor that spurred interest in HPV DNA testing is that cytology-based screening picks up cells from a very specific area on the cervix—the same area where squamous cell cancers are most likely to originate. The glandular-based cancers, or adenocarcinomas, can arise from within the endocervix, which is not typically sampled during the cytology collection procedure. About 80% of cervical cancers are the squamous cell type, which is why cytology has worked well to date. But the incidence of adenocarcinomas has been rising slowly each year despite screening efforts, and the HPV test has the potential to pick up these adenocarcinomas that are largely being missed by cytology.
Not surprisingly, the weight of these data had clinical impact, and cervical cancer screening guidelines began to change. The most significant recent development occurred in March 2012, when a multidisciplinary partnership between the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology released updated screening guidelines stating for the first time that co-testing (a Pap test and an HPV test collected at the same time) is preferred to using Pap cytology alone for screening in women ages 30 to 65. In November of the same year, the nation’s largest OB-GYN organization, the American Congress of Obstetricians and Gynecologists, separately published guidelines that aligned with these recommendations. These organizations have also recognized the value of HPV 16 and 18 genotyping.
With the clinical utility of HPV testing and HPV 16 and 18 genotyping becoming more apparent, an obvious question began to emerge: what if the roles of HPV and Pap were flipped? How would HPV testing work as a primary screen?
Primary screening considerations
One of the factors driving the investigation of a potential primary screening role for HPV DNA testing is its superior sensitivity—greater than 90%, in contrast to roughly 50% for cytology. This high sensitivity is consistent with parameters for disease screening protocols in general: use the most sensitive test for first-line screening and a more specific test for triage of abnormal results.
In cervical cancer screening, the primary goal is to help clinicians with risk assessment and patient management, and thus it is essentially used to identify two groups of patients: (1) women who have low risk and do not need to be tested until the next screening interval; and (2) women who may be at moderate or high risk and need further evaluation to determine the best path of care.
The studies noted above confirmed that HPV DNA testing is very good at finding nearly all of the disease in the population, and that it’s also good at helping with risk management for HPV-positive patients if HPV16 and 18 genotyping is included. The Ronco et al study in particular compared HPV-based screening with cytology-based screening and found that HPV-based screening can reduce cancer incidence.
In addition, recently published baseline data from the ATHENA three-year longitudinal study specifically suggested that HPV genotyping could be considered as a stand-alone test as the primary screen for cervical cancer in women 25 and older.1 In the context of a primary screening role for HPV testing, one of the most compelling insights from the data was related to the predictive ability of a negative test; in other words, if a woman receives a negative HPV result, how confident can she and her physician be that she is “off the hook”?
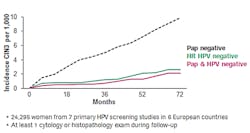
The study by Dillner et al11 found that the HPV-based testing had far better negative predictive ability than cytology-based testing. Only about half of the women who had an HPV negative test ended up developing cervical disease compared to women who had a cytology negative test over the same period of time (Figure 1). So the results from the HPV test provided significantly more valuable information from a disease screening perspective. Co-testing with liquid-based cytology yielded only slightly better predictive ability than HPV testing alone.
What makes these findings about HPV testing particularly compelling in the context of cervical cancer screening is that they address the central question that applies to the vast majority of women being screened: Am I really safe until the next round of screening? The answer suggested by the data is a resounding yes: a woman with a negative HPV test can be twice as certain that she will not develop disease in the next five years as a woman with a negative Pap test can be that she will not develop disease in the next three years.
A Cox et al study suggested that HPV-based screening also offered advantages over Pap-based screening in overall program efficacy.12 The findings indicated that screening women for HPV 16 and HPV 18 genotypes alone would identify a similar number of precancerous cases as the current cytology-based standard of care would, but with substantially fewer women being sent to colposcopy. So even HPV-based screening with this limited scope would potentially improve on the current standard by finding the same amount of disease while decreasing potential harm.
An expanded HPV-based strategy would have even greater benefits. When investigators evaluated a strategy that broadened the screening protocol to include other high-risk genotypes and triaged positive results further by sending to colposcopy, they found it would substantially increase disease detection compared to the current cytology-based standard of care without decreasing efficiency.
Impact on clinical practice
The established evidence for the value of HPV DNA co-testing has already begun to transform clinical guidelines for cervical cancer screening in the U.S. and around the world. The newer data supporting its potential value in a primary screening role have begun to change clinical practice globally as well—with the main drivers being improved cost savings and patient safety by reducing the frequency of testing while simultaneously finding more precancerous lesions that can be treated.
Clinical practice and guidelines in the U.S. have not begun to reflect this data because there is not currently an HPV DNA test approved for primary screening. But outside the U.S., clinical practice has already started to change and many countries are assessing the value of HPV-based screening versus cytology-based screening programs. There are regions in Italy and Latin America that have now moved to HPV-based screening programs, and Mexico and other areas in Europe appear to be moving in that direction as well. There is also interest in countries in Asia where cytology programs are not well established. Australia is currently running a large trial to evaluate primary screening.
Even if an FDA-approved HPV test for primary screening were available in the U.S., clinical adoption of HPV-based screening would likely be a gradual process. Guidelines committees would need to evaluate the data and compare different screening and triage protocol options and make recommendations based on the data.
If guidelines do change, the speed and scope of clinical adoption would still likely vary. Some clinicians are quick to move in the direction of evidence-based medicine if the guidelines go that way. Other clinicians—and their patients—will have a high comfort level with Pap testing and may be hesitant to move quickly to HPV-based protocols even if they think it is the right thing to do based on the data.
For labs, there could be potential impact on testing mix and volume—with HPV DNA testing increasing and liquid-based cytology decreasing. The increase in HPV test demand has already begun to occur with the new guidelines recommending HPV co-testing for women ages 30 to 65. But a more significant shift in practice to HPV testing for primary screening could create a shift in the operational dynamics of a lab that does both cytopathology and molecular diagnostics, for example. The potential changes can serve as an impetus for a lab to develop more diversified capabilities and to investigate options like easy-to-use, automated platforms to simplify cross-training and accommodate increased volume for certain tests.
A board-certified OB-GYN, Ed Baker, MD, FACOG, is Senior Director, Scientific Affairs, for Roche Molecular Diagnostics, a business area of Roche Diagnostics that offers a broad range of PCR-based diagnostic and blood screening assays. He also serves on the faculty of the University of California-Davis Medical School.
- Castle PE, Stoler MH, Wright TC, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol. 2011;12:880-890.
- Herzog TJ, Monk BJ. Reducing the burden of glandular carcinomas of the uterine cervix. Am J Obstet Gynecol. 2007;197:566-571.
ACOG Practice Bulletin No.109: cervical cytology screening. Obstet Gynecol 2009;114:1409-1420.
- Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97(9):675-683.
- Andra B, Kemetli L, Sparén P, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008;100(9):622-629.
- Whitlock EP, Vesco KK, Eder M, Lin JS, Senger CA, Burda BU. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(10):687-697.W214-5.
- Ronco G, Segnan N, Giorgi-Rossi P, Zappa M, Casadei GP, Carozzi F, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98:765-774.
- Rijkaart DC, Berkhof J, Rozendaal L, et al. Lancet Oncol. 2012;13(1):78-88.
- Wright TC, Stoler MH, Sharma A, et al. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol. 2011;136(4):578-586.
- Stoler MH, Wright TC, Sharma A, et al. High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Pathol. 2011;135(3):468-475
- Dillner J, Rebolj M, Birembaut P, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ. 2008;377;a1754.
- Cox JT, Castle PE, Behrens CM, Sharma A, Wright TC, Cuzick J. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. 2013;208(3):184.e1-184.e11.