Blood cancers, also called hematologic malignancies, are the cancers of the blood, bone marrow, and lymph nodes. These cancers can affect anyone, including children. Hematological malignancies account for 9.5% of new cancer diagnoses in the United States.1 According to the Centers for Disease Control and Prevention (CDC), more than 100,000 people in the United States are diagnosed with blood cancers every year, and 50,000 people die from these cancers.2 The availability of advanced and sophisticated molecular diagnostic methods provides answers to questions regarding cancer diagnosis, prognosis, and best course of treatment. This article will outline the types of blood cancers, the known or likely genes causing blood cancers, and a range of in vitro molecular diagnostics laboratory testing methods to detect them.
Types of blood cancers
Hematologic malignancies can be categorized as myeloid neoplasms, lymphoid neoplasms (leukemias and lymphomas), and myeloproliferative disorders.
Myeloid neoplasms
Acute myeloid leukemia (AML) is a cancer of the myeloid cell line which results in the production of monocytic blasts. This leukemia results from production of white cells by the bone marrow that are “stuck” in the cell cycle and cannot mature beyond a certain stage of differentiation.3 There are several subtypes of AML, and the type can dictate a specific treatment related to prognosis.4,5
Diagnosis of AML is made using immunophenotyping and analysis of underlying genetic abnormalities using cytogenetics and molecular testing. AML can occur as an inherited condition or as primary disease, or it may arise as a secondary malignancy following chemotherapy or chemical exposure. Cytogenetic analysis will classify AML patients in one of three risk categories: poor, intermediate, or favorable.3 Molecular testing is now standard of care in intermediate risk AML patients. Analysis of FLT3, NPM1, and CEBPA are recommended by WHO and in the NCCN guidelines. Other subtypes of AML, such as acute promyelocitic leukemia, have specific molecular markers associated with them. Analysis of PML-RARA in APL is now standard of care.6
Chronic myelogenous leukemia (CML) is a clonal disorder of myeloid cell production in the bone marrow. CML is characterized by a specific chromosomal translocation of chromosomes 9 and 22 known as the Philadelphia chromosome. This characteristic chromosomal abnormality can be detected by cytogenetics, by FISH, or by PCR targeted at the BCR/ABL fusion gene.3 CML is treated using one of three tyrosine kinase inhibitor (TKI) drugs.7 TKIs require monitoring of BCR/ABL transcript levels using RT-PCR. This assay measures the amount of BCR/ABL RNA in the patient’s sample. In the clinical laboratory, precautions need to be taken to ensure the validity of an assay result. As RNA is the target material, the lab needs to have procedures in place to extract a high-quality RNA sample as well as procedures to minimize RNA degradation prior to the running of the assay. With the recent availability of a BCR/ABL international standard, labs are now able to compare results. This assay should be able to detect one mutant copy in a background of at least 100,000 normal cells. A highly sensitive assay provides physicians with an early indication of therapy failure or disease reoccurrence.
Despite the fact that TKI therapy was developed as a targeted therapy for CML, not all patients respond to or maintain response to it.7 It has been found that single nucleotide mutations in the BCR/ABL fusion gene can confer resistance to TKI therapy. These mutations have been characterized with regard to their impact on TKI therapy. Patients who fail on front-line TKI therapy should be assessed for BCR/ABL mutations. This data can then guide the treating physician to select an alternative TKI or alternative therapy, such as allogeneic bone marrow transplant.7
Lymphoid neoplasms
Acute lymphoblastic leukemia (ALL) is characterized by the production of immature white blood cells. These cells are continuously dividing, producing additional cells. This overproduction results in normal cells being crowded out of the bone marrow. ALL occurs most frequently in children. A bone marrow examination reveals the presence of the Philadelphia chromosome (the same translocation seen in CML) or a variety of other translocations or complex genetic abnormalities. Immunophenotyping reveals the cells are of lymphoid origin rather than myeloid.3,8 Treatments include chemotherapy, biological agents, and transplant. Great strides have been made in the treatment of ALL in the last 10 to 15 years, and survival rates have increased.1
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in adults.1 CLL is leukemia of the B-cells. In CLL, B-cell production is uncontrolled. The malignant cells accumulate in the bone marrow, crowding out the normal blood producing progenitor cells. Diagnosis of CLL is made by examining smears of the peripheral blood and bone marrow, immunophenotyping, ZAP-70 analysis, and (IgVH) gene mutation status.3 CLL is treated by chemotherapy, radiation therapy, biological therapy using monoclonal antibodies, or bone marrow transplantation.
Lymphomas are a broad group of blood cancers that affect T and B cell lymphocytes. Treatments of lymphomas include chemotherapy, radiation therapy, and bone marrow transplantation. Immunophenotyping of the patient’s cells plays a central role in the diagnosis of a patient with lymphoma. The immunophenotypic analysis will guide molecular test selection such as T-cell clonality analysis, IGVH analysis, or targeted FISH analysis.4
Some of the many types of lymphoma have specific molecular markers: follicular lymphoma/t(14;18) IgH/BCL2; mantle cell lymphoma/t(11;14) IgH/CCND1; marginal zone lymphoma/ t(11;18) API/MALT1; Burkitt lymphoma/t(8;14) IgH/CMYC most commonly or t(2;8) or t(8;22); anaplastic large cell lymphoma/t(2;5) NPM/ALK. There is more molecular research to be done, however, as many lymphomas have not yet been linked to specific markers. These include non-Hodgkin lymphoma (NHL), B lymphoblastic leukemia/lymphoma, T lymphoblastic leukemia/lymphoma, mature B cell lymphoma, small B cell lymphomas, diffuse large B cell lymphoma, plasma cell neoplasms, mature T/NK cell lymphoma, anaplastic large cell lymphoma, and Hodgkin lymphoma.
Myeloproliferative neoplasms
Myeloproliferative neoplasms (MPNs) comprise a group of three distinct yet related myeloproloferative disorders: polycythemia vera, essential thrombocythemia, and primary myelofibrosis.
Polycythemia vera (PV) is a blood disorder in which the patient’s bone marrow makes an excess of red blood cells. It is important to distinguish PV from hemochromotosis, which is a non-malignant disorder of red cell production. PV can occur at any age; however, the disease incidence increases with age, with a median age of onset at 60 years. Patients with PV are at an elevated risk for the development of thrombosis. This risk is caused by the elevated red cell mass, which alters the blood viscosity. The physical exam is non-specific in its findings.
Molecular testing on a peripheral blood or bone marrow sample will reveal the presence of the JAK2 V617F mutation in more than 90% of cases. The JAK2 V617F assay can be run using a variety of assays from allele specific PCR to real time PCR. PV patients negative for the V167F mutation may possess a mutation in exon 12 of the JAK2 gene. This mutation is analyzed by using DNA sequencing of exon 12.3 As is the case in analyzing any acquired DNA mutation, it is important to use an assay with a low level of sensitivity because, as PV is a clonal disease, a mutation-carrying cell may be accompanied by 10, 100, or 1,000 cells negative for the mutation. This type of genetic analysis is vastly different from a typical test for an inherited mutation in which the mutation is present in 0%, 50%, or 100% of cells to be analyzed.
Treatment for PV focuses on reducing the thrombotic risk and reducing the red cell counts of patients. To this end, low-dose aspirin or other anti-platelet agent is given to lower the thrombotic risk. Therapeutic phlebotomy is used to reduce the patient’s red cell mass. An allogeneic bone marrow transplant would be curative; however, the benefits of a transplant rarely outweigh the significant risks to the patient. A specific JAK2 inhibitor is now available, but it currently is not approved for use in PV patients. Additional JAK2 inhibitors are in development and currently in clinical trials.
Essential thrombocythemia (ET) is a blood disorder in which the patient’s bone marrow makes an excess of platelets. ET can occur at any age; however, the disease incidence increases with age, with a median age of onset at around 55 years. Like patients with PV, patients with ET are at an elevated risk for the development of thrombosis. The patient history may reveal bleeding and thrombotic symptoms. Molecular testing on a peripheral blood or bone marrow sample will reveal the presence of the JAK2 V617F mutation in approximately 50% of cases. Mutations in exon 10, (more specifically, mutations in the codons for amino acids 505 and 515) of the MPL gene are diagnostic in JAK2 V617F negative patients. MPL is located at chromosome 1p34 and encodes for the thrombopoietin receptor.3
As is the case with PV, treatment for ET focuses on reducing the thrombotic risk to patients; low-dose aspirin or other anti-platelet agents are given. Platelet apheresis can be used to reduce the patient’s platelet count. Drugs such as hydroxyurea or interferon-a can also be used to reduce the production of platelets in ET patients.
Primary myelofibrosis (PMF) is a blood disorder in which the patient’s normal bone marrow becomes acellular and fibrotic. The fibrous tissue squeezes out the normal blood-producing cells, resulting in a progressively worsening pancytopenia. PMF can occur at any age; however the disease incidence increases with age, with a median age of onset at around 45 years. As with ET, molecular testing on a peripheral blood or bone marrow sample will reveal the presence of the JAK2 V617F mutation in approximately 50% of cases. Mutations in exon 10 of the MPL gene are diagnostic in JAK2 V617F negative patients.
Treatment for PMF focuses on restoring the hematopoietic (blood-producing) capability to patients. To this end, transfusions and folic acid are used. Drugs such as dexamethasone, hydroxyurea, or interferon-a can be used in PMF patients. PMF is a progressive disease, and a bone marrow transplant would be curative, as is the case with PV; again, however, a transplant poses serious risks to the patient. The FDA approved the drug ruxolitionib, a JAK2 inhibitor, in November 2011 for the treatment of PMF. Clinical trials showed the drug improved overall survival in PMF patients compared to placebo.
The myelodysplastic syndromes (MDS) are clonal diseases of hematopoietic stem cells which result in cytopenia(s) and abnormal cell production in the myeloid cell lines. Patients with MDS possess a risk for later development of AML. MDS occurs primarily in older adults with a median age of onset at 70 years. The assessment of a bone marrow specimen is the chief diagnostic approach in MDS. Bone marrow specimens are evaluated for cellularity, immunophenotyping, cytogenetics, and molecular studies. Cytogenetic and molecular studies have a major role in determining prognosis in MDS patients. MDS patients can be stratified in three risk categories: favorable, intermediate, and poor. Patients are stratified based on blast counts in the marrow, presence of cytogenetic abnormalities, and cell counts.3
Several papers have described the role of TP53 mutations in MDS. TP53 is a critical tumor suppressor protein that regulates the cell cycle. Mutations in TP53 can disrupt the protein’s protective capability and permit the development of tumors, including MDS among others.9
Molecular diagnostic methods
Molecular diagnostic tests detect mutations in DNA or RNA that are associated with blood cancers. In vitro molecular diagnostics requires three basic steps:
- The extraction and purification of nucleic acids from blood or bone marrow samples. Labs performing molecular testing for analysis need to have policies and procedures in place to assess the quality and concentration of extracted DNA or RNA. The purity of extracted DNA can be estimated from the A260/A280 ratio. An A260/A280 ratio between 1.7 and 2.0 generally represents a high-quality DNA sample, and pure RNA has an A260/A280 of 2.1.
- The amplification of the nucleic acid of interest (targeted cancer genes) should be assessed to ensure the intended target has been amplified.
- The detection of the amplified target using appropriate techniques.
The Federal Drug Administration (FDA),10 Centers for Medicare & Medicaid Services,11 and Clinical Laboratory Improvement Act (CLIA)12 regulate clinical molecular tests like all other clinical laboratory tests. As per the regulations the molecular tests for blood cancers can be categorized in several ways:
- FDA approved/cleared tests. The FDA approved tests can be of two categories: nucleic acid-based tests andcompanion diagnostic tests. FDA-cleared molecular tests may be modified by a laboratory.
- Analyte specific reagents (ASRs) are FDA-regulated and can be used for patient specimens after in-house validation. The components of the assay cannot be sold in a “kit” form or purchased from the same manufacturer.
- Research use only (RUO). This term usually refers to tests and rarely to devices. The assays are not FDA regulated; however FDA defines the category as research, not diagnosis. Laboratories buy products labeled as RUO and then use the results in generating diagnoses.
- Laboratory developed tests (LDTs). These tests are not FDA-regulated, but they must be validated using CLIA standards before they can be used to generate clinically used results.
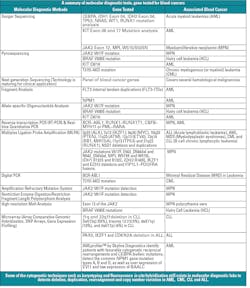
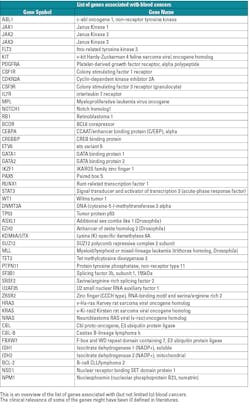
There are several molecular diagnostic techniques to detect blood cancers. Each technique has some advantages and disadvantages. The following shows a detailed summary (in table form) of the established molecular diagnostic methods 5,6,13 to detect known genes associated with blood cancers.
The future of mol dx in blood cancers
The molecular diagnostics of blood cancer is growing rapidly. With the evolution of innovative technology such as next generation sequencing (NGS), it is likely to become even more prominent as a practice in testing.
NGS, also called massively parallel sequencing (MSP), is one of the most significant technological advances in recent years. Medical research has embraced the technology in the cancer field, and efforts are being made to catalogue mutations in multiple cancer types. That will lead to new discoveries and become translated to new diagnostic, prognostic, and therapeutic targets. NGS is now maturing to the point where many laboratories are considering it for routine diagnostic use. The sensitivity, speed, and reduced cost per sample make it highly attractive.
As there will be identification of more genetic determinants for cancer, there will be greater need to adopt multigene assays that can quickly and reliably sequence complete genes from individual patient samples. It is likely that whole exome sequencing and whole genome sequencing will be the future trends in molecular diagnostics of blood cancers, at the cost of putting single-gene tests at risk.
D.P. Dash, PhD, is a Scientific Director of the Molecular Oncology Laboratory at BloodCenter of Wisconsin. His research/professional interest is on the development and implementation of new technologies and novel assays that promise to improve the ability to diagnose blood cancer in a rapid and efficient manner. He is particularly interested in assessing the utility of these technologies to discover how they can best be used to improve patient care and impact physician decision making. Michael Janasik is a Product Manager for the Diagnostic Laboratories at BloodCenter of Wisconsin. His professional focus is on the implementation of new technologies or strategies to diagnose and treat individuals with blood-related disorders.
References
- The Leukemia and Lymphoma Society. Facts & Statistics. http://www.lls.org/diseaseinformation/getinformationsupport/factsstatistics. Accessed July 2, 2013.
- Centers for Disease Control and Prevention. http://www.cdc.gov. Accessed July 2, 2013.
- Sheehan AM. Introduction to the WHO classification of hematologic malignancies. ISS Refresher Course, Baylor College of Medicine.
- Gibbs JN. Regulatory pathways for molecular diagnosis. Detailing the various options available and what each requires. GEN. 2008:28(14).
- Mason J, Griffiths M. Molecular diagnosis of leukemia. Expert Rev Mol Diag. 2012;12(5):511-526.
- National Comprehensive Cancer Network, NCCN Guidelines: Acute Myeloid Leukemia, Version 2.2013. www.nccn.org. Accessed July 2, 2013.
- National Comprehensive Cancer Network, NCCN Guidelines: Chronic Myelogenous Leukemia, Version 2.2013. www.nccn.org. Accessed July 2, 2013.
- National Comprehensive Cancer Network, NCCN Guidelines: Acute Lymphoblastic Leukemia, Version 2.2013. www.nccn.org. Accessed July 2, 2013.
- National Comprehensive Cancer Network. NCCN Guidelines: Myelodysplastic Syndromes, Version 2.2014. www.nccn.org. Accessed July 2, 2013.
- U.S. Food and Drug Administration. http://www.fda.gov. Accessed July 2, 2013.
- Centers for Medicare & Medicaid Services. http://www.cms.gov. Accessed July 2, 2013.
- Centers for Disease Control and Prevention. Clinical Laboratory Improvement Amendments (CLIA). http://wwwn.cdc.gov/clia. Accessed July 2, 2013.
- National Cancer Institute (NCI). http://www.cancer.gov. Accessed July 2, 2013.