Urinalysis is a valuable tool used to diagnose and monitor renal and urinary tract illnesses. Typically it is a moderate- to high-sample volume test for a general chemistry lab, representing up to 30% of all samples received. Routine urinalysis consists of macroscopic examination, chemical analysis, and microscopic urine sediment examination. Table 1 summarizes each element and its potential clinical significance.
CONTINUING EDUCATION
To earn CEUs, visit mlo-online.com and see this month’s test by clicking the CE menu item.
LEARNING OBJECTIVES Upon completion of this article, the reader will be able to:
- Review interpretation of urinalysis findings.
- Identify the automated methods for urine sediment identification discussed in this article.
- Describe methodology used for the automated urinalysis sediment exam.
- Compare advantages and disadvantages of manual microscopy and automated systems.
Chemical analyses are often performed using reagent strips that are introduced to the urine sample. Color changes are read manually or by strip readers utilizing reflectance photometry. Alternatively, most elements can be quantified more precisely using chemical autoanalyzers, pH probes, and osmometers.1,2 The microscopic examination is traditionally performed manually on sediment from a centrifuged urine sample. This process is both time-consuming and labor-intensive. Moreover, it requires advanced technologist training for accurate interpretation, and can be subject to errors due to incomplete pelleting or degradation of formed elements during centrifugation or sample storage.
The guidelines for urinalysis published by the Clinical and Laboratory Standards Institute3 state that each lab should decide when to perform microscopic examination of urine sediment based on its patient population. Considerations include physician preference and laboratory protocols regarding specific patient populations. Including manual microscopic screening on all routine urinalysis samples is not practical for most labs due to the substantial workload this entails. Therefore, some laboratories have adapted a two-step procedure, using dipstick tests as a screen to exclude urine samples from further manual microscopic analysis. Studies that compared automated complete urinalysis to this two-step approach have reported that the complete automated approach yielded improved sensitivity (98% vs. 91%) and negative predictive value (96.5% vs. 66.5%) for a pathologic urine sediment finding.4 Consequently, there is a shift toward automating routine urinalysis not only in large reference laboratories but also in smaller laboratories, since standardization, improved patient care, and even cost savings can all justify the up-front expense.
Urinalysis automation
In a traditional urinalysis, 12 mL of urine is centrifuged for 5 minutes at 1400 rpm. The resulting pellet is resuspended in 250 μL of urine. Approximately one drop is analyzed on a slide under a microscope, and observed elements are quantified as the number per high power field. More exact quantification has been described using a hemocytometer,5 but this is rarely employed today.
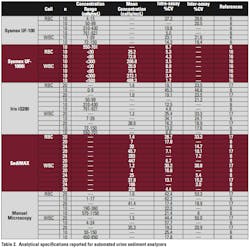
Manual microscopy can take up to six minutes per sample compared to less than one minute for automated systems. Hence, labor needs are less and turnaround time often is dramatically reduced. Each instrument can be combined with automated test strip analyzers to fully automate routine urinalysis. The authors are aware of three automated urine sediment analysis systems that have been evaluated in the literature. The analytical and technical specifications are listed in Table 2 and Table 3.
Each system uses a different technology to classify and quantify urine sediment particles and offers an improvement in standardization over manual microscopy by eliminating potential inter-technician variability during slide interpretation. Quality of results may also be improved since samples can be processed more efficiently and therefore closer to their collection time, decreasing the likelihood of cell lysis, microbial contamination, or precipitation from prolonged storage. Each method is described below. Their advantages and disadvantages are summarized in Table 4.
Flow cytometry: Sysmex previously offered the UF-100 system that employed flow cytometry and fluorophores to categorize cells and casts in uncentrifuged urine labeled with fluorophores according to their fluorescence, size, impedance, and forward scattered light. In 2007, it introduced the FDA-cleared UF-1000i, a new generation of particle analyzer that provides counts for WBCs, RBCs, epithelial cells, and bacteria with high precision. Sensitivity for pathological casts, crystals, yeast, sperm, mucus, and abnormal cells are also increased, but specificity is still poor for differentiating these elements, which therefore must be confirmed by manual microscopy. Users may specify criteria and thresholds for results to be flagged for manual microscopy offline. The results are displayed as scattergrams that can be saved and viewed by the operator.
Digital imaging with particle recognition: The Iris iQ200 from Iris Diagnostics uses digital imaging and Auto-Particle Recognition (APRTM) to classify and quantify urine particles in uncentrifuged urine based upon size and shape. The analyzer classifies images into categories including RBC, WBC, WBC clumps, hyaline casts, unclassified casts, EC, nonsquamous EC, bacteria, yeast, crystals, mucus, sperm, and amorphous substances. Digital images of particles are collected, ~500 frames per sample, and can be viewed and reclassified by the operator, potentially without the need for manual microscopy. However, many labs still pull samples with pathological findings for a secondary full manual microscopy. Since the sample is unstained, the appearance of components differs from that observed by manual phase contrast microscopy, necessitating considerable technician retraining.6
Automated microscopy with digital imaging: A newer automated urine sediment analyzer called sediMAX (77 Elektronika, distributed in Europe and Asia by A. Menarini Diagnostics) has been introduced and compared to manual microscopy. The instrument produces a monolayer of urine sediment by centrifugation in a special cuvette. The sediment is analyzed by a bright field microscope and digital camera to capture and categorize 15 particle images based upon size and shape using image processing software. The manufacturer strives to continuously improve the software and its performance, and these software upgrades are made available to existing instruments.7 Digital images can be viewed by the operator with zoom capability.8 Overall, compared to manual microscopy the instrument performed well for detecting RBCs and WBCs (>80% sensitivity), but not as well for pathological casts and nonsquamous epithelial cells (~50% sensitivity).
Urine culture screening
Sensitive and specific methods for detecting bacteria are particularly valuable in decreasing the number of samples sent to the microbiology lab for culture to confirm the diagnosis of urinary tract infections (UTIs). Up to 70% of specimens submitted to a typical hospital lab are negative, constituting a large resource utilization that could potentially be reduced. One study using the Sysmex UF-100 and a bacterial cutoff of 3,000/μL for screening urine samples demonstrated a sensitivity of 94.4% and specificity of 73.4% for subsequent culture positivity (>105 organisms/mL) with a 0.012% false negative rate.9 Thus it was possible to eliminate 58.2% of samples sent for culture. Similar performance has been demonstrated for the UF-1000i,10 Iris iQ200,11 and SediMAX.12 Each lab will need to establish its bacterial cut-offs that trigger subsequent culture, and this might vary depending upon the population being screened.
Fully automated urinalysis
The three urine sediment analyzers described are capable of interfacing with automated strip analyzers to produce comprehensive routine urinalysis reports. Both physicochemical and microscopic examination appear necessary for effective screening purposes, and use of a fully automated system allows this to be accomplished while avoiding the cumbersome and labor-intensive process of manual microscopy.
A 2006 CAP Q-Probes study (n = 11,243 urinalysis tests in 88 institutions) reveals 14.9% use automated microscopic urinalysis analyzers (relatively evenly split between Iris and Sysmex).13 In various labs, manual microscopy was triggered by an abnormal macroscopic appearance of urine, a physician request, or virtually any positive urinalysis result. Reflex testing algorithms were reported by 93.1% of labs triggered by dipstick results. Manual microscopic urine sediment evaluations were performed at a median rate of 31% in laboratories that utilized automated microscopic analyzers while those that didn’t had double that rate at 64%.
Advantages of automated urinalysis are not restricted to larger high-throughput labs. There are several reports of smaller hospital labs that successfully switched to automated platforms, including a 350-bed hospital in Alaska that performs ~120 urinalyses per day. Within eight months of using the Iris iQ200 system, it reportedly paid for the instrument from savings on consumable products and relocating one FTE technologist.14 Labs utilizing automated urinalysis note that producing digital reports is less time-consuming and less prone to clerical errors. Clinicians also often appreciate the quantitative report and precise cut-offs established by the lab for their patient population when making treatment decisions, as opposed to the qualitative or semi-quantitative 0, 1+, 2+ system often used for dipsticks and manual microscopy.
Automated urinalysis offers the benefits of convenience, efficiency, and increased sensitivity for detecting renal and urinary tract abnormalities. The true value may lie in the ability to efficiently screen and report out urine samples that lack pathologic findings. In our experience at a large clinical lab, up to 70% of urine samples can thus be screened and reported without the need for a confirmatory manual microscopy, saving a considerable amount of time and labor. All of the automated sediment analyzers reviewed have comparable performance characteristics with improved standardization over manual microscopy. The system a lab chooses can be based on individual preference for the advantages each has to offer.
References
- de Buys Roessingh A, Drukker A, Guignard JP. Dipstick measurements of urine specific gravity are unreliable.Archives of Disease in childhood. 2001;85(2):155-157.
- Desai RA, Assimos DG. Accuracy of urinary dipstick testing for pH manipulation therapy. Journal of Endourology/Endourological Society. 2008;22(6):1367-1370.
- Clinical and Laboratory Standards Institute, Urinalysis: Approved Guideline—3rd edition. 2009.
- Shayanfar N, Tobler U, von Eckardstein A, Bestmann L. Automated urinalysis: first experiences and a comparison between the Iris iQ200 urine microscopy system, the Sysmex UF-100 flow cytometer and manual microscopic particle counting. Clinical Chemistry and Laboratory Medicine. 2007;45(9):1251-1256.
- Lin DS, Huang FY, Chiu NC, et al. Comparison of hemocytometer leukocyte counts and standard urinalyses for predicting urinary tract infections in febrile infants.The Pediatric Infectious Disease Journal. 2000;19(3):223-227.
- Mayo S, Acevedo D, Quiñones-Torrelo C, Canós I, Sancho M. Clinical laboratory automated urinalysis: comparison among automated microscopy, flow cytometry, two test strips analyzers, and manual microscopic examination of the urine sediments.Journal of Clinical Laboratory Analysis. 2008;22(4):262-270.
- Barta Z, Kránicz T, Bayer G. UriSed Technology –A standardized automatic method of urine sediment analysis. European Infectious Disease. 2011;5(2):139-142.
- Zaman Z, Fogazzi GB, Garigali G, et al. Urine sediment analysis: analytical and diagnostic performance of sediMAX – a new automated microscopy image-based urine sediment analyser.Clinica Chimica Acta; International Journal of Clinical Chemistry. 2010;411(3-4):147-154.
- Kim SY, Kim YJ, Lee SM, et al. Evaluation of the Sysmex UF-100 urine cell analyzer as a screening test to reduce the need for urine cultures for community-acquired urinary tract infection. American Journal of Clinical Pathology. 2007;128(6):922-925.
- Broeren MA, Bahceci S, Vader HL, Arents NLA. Screening for urinary tract infection with the Sysmex UF-1000i urine flow cytometer. Journal of Clinical Microbiology. 2011;49(3):1025-1029.
- Luciano R, Piga S, Federico L, et al. Development of a score based on urinalysis to improve the management of urinary tract infection in children.Clinica Chimica Acta; International Journal of Clinical Chemistry. 2012;413(3-4):478-482.
- Falbo R, Sala MR, Signorelli S, et al. Bacteriuria screening by automated whole-field-image-based microscopy reduces the number of necessary urine cultures.Journal of Clinical Microbiology. 2012;50(4):1427-1429.
- Tworek JA, Wilkinson DS, Walsh MK. The rate of manual microscopic examination of urine sediment: a College of American Pathologists Q-Probes study of 11,243 urinalysis tests from 88 institutions.Archives of Pathology & Laboratory Medicine. 2008;132(12):1868-1873.
- Paxton A. Automation nudges urinalysis into spotlight. CAP Today. 2007.
- Burtis CA, Ashwood ER, Bruns DE, eds. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. St. Louis, MO: Elsevier Saunders; 2006.
- Wang J, Zhang Y, Xu D, Shao W, Lu Y. Evaluation of the Sysmex UF-1000i for the diagnosis of urinary tract infection. American Journal of Clinical Pathology. 2010;133(4):577-582.
- Akin OK, Serdar MA, Cizmeci Z, Genc O, Aydin S. Comparison of LabUMat-with-UriSed and iQ200 fully automatic urine sediment analysers with manual urine analysis. Biotechnology and Applied Biochemistry. 2009;53(Pt 2):139-144.
- Jiang T, Chen P, Ouyang J, Zhang S, Cai D. Urine particles analysis: performance evaluation of Sysmex UF-1000i and comparison among urine flow cytometer, dipstick, and visual microscopic examination.Scandinavian Journal of Clinical and Laboratory Investigation. 2011;71(1):30-37.
- van den Broek D, Keularts IM, Wielders JP, Kraaijenhagen RJ. Benefits of the iQ200 automated urine microscopy analyser in routine urinalysis.Clinical Chemistry and Laboratory Medicine. 2008;46(11):1635-1640.
- Boven LA, Kemperman H, Demir A. A comparative analysis of the Iris iQ200 with manual microscopy as a diagnostic tool for dysmorphic erythrocytes in urine.Clinical Chemistry and Laboratory Medicine. 2012;50(4):751-753.
Darci R. Block, PhD, DABCC, co-directs the Central Clinical Laboratory and Central Processing and Laboratory Services in the Department of Laboratory Medicine and Pathology at Mayo Clinic in Rochester, MN.
John Lieske, MD, is Professor of Medicine, and Director of the Renal Function Laboratory in the Department of Laboratory Medicine and Pathology at the Mayo Clinic. Currently he is Principal Investigator for the NIH-funded Mayo Clinic O’Brien Urology Research Center and Assistant Director for the Rare Kidney Stone Consortium.