D
uring disasters, emergency medical responders deploy to
disaster sites.1 They carry
point- of-care testing (POCT) devices,
such as oxygen-saturation monitors
(pulse oximeters), blood-glucose meters,
and other small hand-held devices.1
Disaster medical-assistance
teams (DMATs), international medical-surgical response teams
(MSuRTs), and
other first responders typically encounter harsh environmental conditions,
temperature extremes, and high humidity. In a recent study conducted by Dr.
Louie and colleagues at our center at the University of California-Davis,
results indicated, however, that glucose test strips and blood-gas
cartridges may not be able to withstand harsh environment conditions often
encountered at disaster sites and noted, “The performance of glucose meter
test strips and blood-gas analyzer cartridges was affected adversely and,
sometimes inconsistently, by thermal stresses.”2 Without durable
and robust POCT equipment, diagnosis and treatment of victims at disaster
sites becomes increasingly complicated. The article further urges, “DMATs
and emergency medical responders should be aware of the potential risks of
inaccurate results from POCT when operated in adverse conditions.”2
Additionally, the need for robust POC devices and
reagents was clearly evident in the 2004 tsunami in Southeast Asia and
Hurricane Katrina in the United States. The tsunami claimed more than
310,000 lives, with Thailand suffering a loss of nearly $1 billion.1
Similar losses were observed with Hurricane Katrina. In the United States,
an estimated 1,577 lives were lost, thousands were left homeless, and
billions of dollars in damages resulted.1
Regional catastrophes like these “newdemics”3,4
lead to sequential magnified setbacks; and, typically, communities lack the
POCT resources to effectively handle the respective disaster situations.1,3
These disasters highlight the need for new sturdy, hand-held, and robust POC
technologies capable of effectively operating in disaster situations. Soon,
the National Institute of Biomedical Imaging and Bioengineering (NIBIB)
aided by the National Institutes of Health (NIH) will provide a solution to
this gap in POCT resources.5 Better prepared first responders
will carry reliable diagnostics to the point of care, wherever it might be.
The potential for POCT — testing done at or near the site
of the patient — to positively impact the manner in which healthcare is
delivered in the United States was the result of a workshop sponsored by
NIBIB in 2006.5 The workshop recommended the formation of
multidisciplinary research collaborations to facilitate clinical testing
early in the developmental process.5 In September 2007, the NIBIB
established and funded four centers through the NIH cooperation agreement
funding mechanism known as U54.5 Each research center focuses on
a specific healthcare-delivery theme (see Table 1).5
The goal of the POCT centers is to address unmet clinical
needs in POCT by utilizing the creation and collaboration of the
multidisciplinary partnerships to build expertise in the development of
integrated systems.5 The centers work independently to achieve
their particular research goals and collectively as part of a national
network to allow coordinated development, clinical evaluations, and
reduction to practice of new POC devices.5
Five core functions organize the center's activities.
Core One focuses on conducting in-house clinical testing of prototype POC
devices.5 Core Two utilizes the collaboration with physical
scientists, biochemical scientists, computational scientists, and
bioengineers on exploratory projects.5 Core Three addresses
clinical needs assessment in areas anticipated to advance the field of POCT
and disseminating this information to the community via education.5
Core Four provides training to technology developers on clinical issues
related to the development of POC devices.5 Finally, Core Five
outlines the administrative structure in order to ensure that each of the
POCT centers achieves both individual and shared goals.5
POCT serves as a critical component of acute disaster
response and follow-up recovery; but, at present, POCT devices available in
the consumer market and for routine use do not meet adequate standards for
disaster conditions. Our POCT center at UC Davis-LLNL specifically focuses
on pathogen detection and development of devices capable of withstanding
harsh disaster conditions. In addition, we utilize clinical needs assessment
as an effective tool for gathering information on POC devices, test
clusters, and pathogens considered critical for diagnosis at a specific
disaster site.
To facilitate our development of rapid pathogen
detection, a Clinical Needs Assessment Survey based on what we call “visual
logistics” has been developed and is available by teleconference, in print
upon request, and online through Survey Monkey (see Table 2). Responses
serve as a guide to specifically identify pathogens that clinicians, several
of whom are disaster experts, believe are vital to diagnosing and treating a
patient effectively, given each disaster scenario. Through Clinical Needs
Assessment, our center plans to develop a pathogen detection device that
will give fast, rapid diagnosis and enable better patient care at a disaster
site. Our Clinical Needs Assessment Survey asks questions covering the
spectrum from device design to pathogen test clusters in the differential
diagnosis.
Table 2. Survey Monkey instructions
Please go to
www.surveymonkey.com/s.aspx?sm=9SYx6nymRDH4ed9EO_2b_2bl1w_3d_3d .
Follow the instructions on the screen to complete the survey. Note: Your progress will be saved after pressing the “next” button at the end of each page. Please note, your progress online is managed through your web browser cookies, and your identity is maintained using your IP address. For this reason, please try to complete the survey on the same computer and do not delete the cookies on your web browser prior to completion of the online survey.
Thank you for your time and input on the survey! Please contact, Keith Brock, Research Specialist, at 530-753-4702, e-mail [email protected], for any comments or questions to better facilitate your participation in the Clinical Needs Assessment Survey.
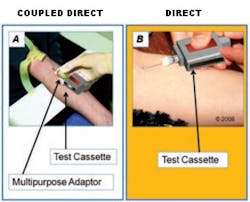
Figure 1 asks the interviewee about test
cassette sampling methods, specifically whether the sampling needle needs to
be on the test cartridge or on a standard vacutainer. Figure 2 inquires
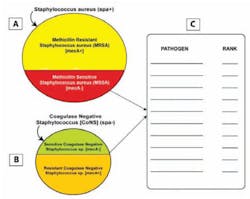
about who should be trained in POCT for disasters. Figure 3 evaluates a
pathogen test cluster design for Staphylococcus aureus. The
interviewee must consider whether to detect coagulase negative
Staphylococcus when ruling out methicillin-resistant Staphylococcus
aureus (MRSA).
In addition, the pathogens for consideration are ranked
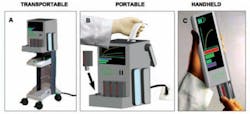
in order of importance when diagnosing MRSA (Figure 3). Figure 4 evaluates
which type of device would be preferred in various settings (i.e., disaster,
urgent-care, or emergency-room settings). The knowledge gained by our POCT
center from the survey directly guides the development of pathogen detection
devices.
Participation in the survey gives MLO readers the
opportunity to join our center in an effort to raise the standards of
patient care available at any disaster site. The global problem of disaster
response and preparedness affects everybody, including laboratory
professionals and supervisors, who often are seasoned experts in the tests
performed by POCT.
The survey is available online (Table 2 provides
instructions) and can be completed in about 30 minutes; the hyperlink
directs you to the online survey. Please take our survey and help bring
adequate and effective care to the site of patient regardless of where
disasters may strike.
POCT devices need to be suitable for response site
environmental conditions beyond equipment storage and operating limits.2
POCT devices carried by first responders need to endure these environmental
stresses to avoid producing inaccurate test results.2 Patient
care available at these sites represents a top priority. Each of the POCT
centers individually and collectively continues striving to bring POCT
technologies to the frontline in order to make global health a world
reality.5
Correctly utilized and executed, POCT allows for fast
on-site testing that facilitates evidence-based decisions.3,4
POCT developed poorly, not explored fully, or not deployed proactively to
meet challenges at disaster sites, such as Hurricane Katrina or the tsunami
in Thailand,1-4 detracts from on-site care. In order to prevent
this from occurring, the devices taken to any of these respective field
sites have to be able to withstand environmental conditions encountered and
must be tailored for specific use by personnel working in these conditions.3,4
Some POCT devices, however, may be able to effectively
handle adverse environmental conditions. Preliminary results from thermal
stress testing of a point-of-care hemoglobin- measurement system show
promising results.6 The potential to revolutionize the current
POCT field is here. Join us to take disaster response and preparedness to a
heightened level by participating in our survey. Collectively, we can assure
that patient care will be available at disaster sites, that is, at the point
of need.
Kristin N. Hale, BS, BA, is a graduate student researcher and currently in the graduate program for
Comparative Pathology at the Univeristy of California-Davis (UC-D) with her
undergraduate degrees awarded there. T. Keith Brock, BS,
is a UC-D graduate and research specialist there. Gerald J. Kost, MD, PhD,
MS, FACB, is the
director of the Point-of-Care Testing Center for Teaching and Research
(POCT•CTR), and is a colleague of Hale and Brock.
References
- Kost GJ, Tran NK, Tuntideelert M, et al. Katrina, the
tsunami and point-of-care testing: optimizing rapid response diagnosis
in disasters. Am J Clin Pathol. 2006;126:513-520. - Louie RF, Sumner SL, Belcher S, et al. Thermal stress
and point-of-care testing performance: suitability of glucose test
strips and blood gas cartridges for disaster response. Disaster Med
public Health Preparedness. 2009;3:13-17. - Kost GJ, Minear M, Siegel PM, et al. Knowledge,
education, mind connectivity: using telemedicine to achieve a global
vision for point-of-care testing. Point of Care. 2008;7(2):69-71. - Kost GJ. Newdemics, public health, small-world
networks, and point-of-care testing. Point of Care.
2006;5:138-144. - Kost GJ, Korte B, Beyette FR, et al. The NIBIB point
of care technologies research network center themes and opportunities
for exploratory POC projects. Point of Care. 2008;7:41. - Kraynov L, Brock T, Louie R, et al. Effects of
thermal stress on reagent test strips and test cuvettes for
point-of-care glucose, hemoglobin, and white blood cell measurements. 20th
Annual Undergraduate Research, Scholarship, and Creative Activities
Conference at UC Davis. Davis, CA. March 2009.
Acknowledgement
The project described was supported by Award Number
U54EB007959
(Dr. Kost, PI) from the National Institute of Biomedical Imaging and
Bioengineering. The content is solely the responsibility of the authors and
does not necessarily represent the official views of the National Institute
of Biomedical Imaging and Bioengineering or the National Institutes of
Health. Table and figures provided courtesy and permission of knowledge
optimization,® Davis, CA.
Figure 1. Test cassette sampling method coupled direct vs. direct
Pictorial representation of coupled direct cassette sampling method (panel A) and direct cassette sampling method (panel B). Panel A illustrates a test cassette sampling from a multipurpose adaptor. Panel B illustrates another test cassette which includes a built in needle for direct sample collection.
Figure 2. Class of POCT user
The pictorial representation displays the various operators of POCT devices. Potential personnel to operate POCT include medical technologists (Panel A), nurses (Panel B), first responders (Panel C), and Patients (Panel D).
Figure 3. Pathogen test cluster design for Staphylococcus aureus
The illustration depicts possible pathogens used for detection of methicillin resistant Staphylococcus aureus (MRSA). The image is meant to address whether coagulase negative Staphylococcus (CoNS) is considered when ruling out MRSA for pathogen detection.
Figure 4. Device design format
The image represents various device design formats. Panel A represents a transportable device on a cart that is AC powered. Panel B displays a portable, electrical outlet-powered bench top device with a handle for carrying. Panel C illustrates a small, battery operated, handheld device.