CE
CONTINUING EDUCATION
To earn CEUs, see current test at
www.mlo-online.com
under the CE Tests tab.
LEARNING OBJECTIVES
Upon completion of this article, the
reader will be able to:
- Understand the traditional means of assessing breast-cancer risk,
and explain their shortcomings in identifying patients who could benefit
from preventive approaches. - Describe the longstanding cytomorphology of breast specimens (breast
epithelial aytpia biomarker) as it relates to the Papanicolaou or “Pap”
technique. - Discuss the state of technology for breast-fluid collection.
- Discuss best practice for nipple aspirate fluid (NAF) specimen
acquisition, transport, and labeling, as well as slide preparation and
staining, and slide screening and interpretation. - Describe the recommended classification system for reporting NAF
results.
The current
standard for screening women to assess their breast health and risk of
developing breast cancer is review of family history, physical breast exam,
plus regular mammography for women over 40. Clinical experience has shown,
however, that none of these methods is very effective in identifying cancers
at their earliest stages. By the time the cancer is detected by either
physical examination or mammography, the woman may have had the disease for
years, with significant risk of serious consequences — including death.
There is a growing need to identify those women who are at increased risk
for developing breast cancer and focusing resources on prevention strategies
and earlier detection for these women.
It is generally recognized that benign
intraepithelial lesions of the breast ductal system is a biomarker with
the closest biologic association to increased risk of developing breast
cancer. Previous studies of open breast biopsies have demonstrated that
the presence of atypical epithelial cells is associated with a four- to
fivefold risk of developing future breast cancer.1 Multiple
long-term studies show that the identification of atypical epithelial
cells found in cytology specimens, such as nipple aspirate fluid (NAF)
or fine-needle aspiration (FNA), is also associated with an elevated
risk of developing breast cancer, as was observed with open biopsies.2,3
Today, cytologic examination could be used to identify a subgroup of
asymptomatic women who are at increased risk for breast cancer.4,5
The majority of these women have no established risk factors for the
development of breast cancer. The finding of atypia can alert the
treating physician that further surveillance and, in selected cases,
preventive efforts may be indicated.
The advent of new acquisition techniques over the
past decade makes breast sampling more accessible for healthcare providers
and more acceptable for patients. The emergence of these new methods to
collect breast samples has meant that laboratories and cytology
professionals need to be knowledgeable in the examination of exfoliative
cytology specimens of the breast. The rationale for breast-health screening
using cytology is explored here, along with a review of collection methods
and guidelines for interpretation and reporting of such specimens.
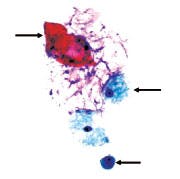
Category O. Negative for atypical or malignant cells. Rare
benign (non-hyperplastic) ductal epithelial cell (arrow). Foam cells
and proteinaceous debris noted in the background (arrow). The
squamous epithelial cell (arrow) is a contaminate from the breast or
nipple surface. ThinPrep-Pap stain.
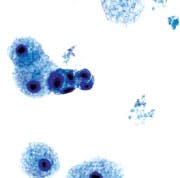
Category I. Benign — normal ductal epithelial cells
identified. Foam cells with small cluster of ductal epithelial
cells. ThinPrep-Pap stain.
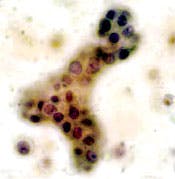
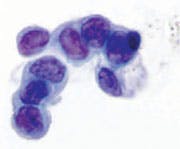
Category II. Benign — hyperplastic ductal epithelial cells
identified. Note the slight nuclear enlargement and fine, smooth
chromatin, which is evenly distributed. Nucleoli are sometimes
present. Millipore Filter-Pap stain.
The need for an effective risk screen
While progress has been made in detecting and
treating breast cancer, the disease remains, other than skin cancer, the
most common cancer for women in the United States. It accounts for about 26%
of all cancers found in U.S. women. An estimated 182,460 cases of invasive
breast cancer were expected to be found in 2008, with 40,480 women dying
from it.6
Despite the high total number of deaths,
breast-cancer death rates have been moving downwards in recent years.
Improved detection methods (e.g., regular mammograms) are credited with much
of the decline in mortality rates, because they help find tumors before
those can be felt during a manual breast exam by a physician or a woman
herself. Earlier detection means the tumor may be smaller and more
treatable, with fewer consequences. But mammograms are not very effective in
women with dense breasts, and they are not very helpful if the goal is
breast-cancer prevention. Mammograms are not useful for identifying patients
who have a significant risk of developing the disease in the future and who
might, thus, benefit from preventive care or more frequent observation.
Few women younger than 40 get regular mammograms, yet
they are at much greater risk of dying from the disease should they contract
it. This is a cohort that would especially benefit from risk screening.
Women in the 40 to 50 age range are also prime candidates for risk
screening. Although mammograms are widely recommended for women 40 and
older, they are not as effective in women under 50, because high breast
density interferes with the detection of abnormalities.
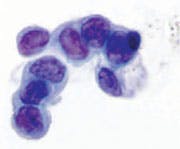
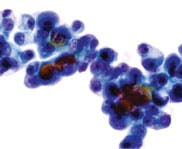
Figure 6. NAF Category IV. Suspicious for malignancy. Group of highly atypical ductal epithelial cells with dark, hyperchromatic chromatin. The chromatin is more irregular in its distribution, and coarsened. Note the nuclear contour pleomorphism is greater. There are prominent nucleoli. Millipore Filter-Pap stain.
In recent decades, more effective means of preventing
breast cancer have been developed for women known to be at high risk for the
disease. The less aggressive options include increased surveillance (e.g.,
more frequent clinical breast exams and breast self-exams); lifestyle
changes regarding diet and exercise; and use of enhanced imaging modalities.
In some cases, more aggressive means such as chemoprevention (e.g.,
Tamoxifen) may be used. The National Surgical Adjunct Breast and Bowel
Project (Breast Cancer Prevention Trial) showed that administration of
tamoxifen reduced the risk for invasive and non-invasive breast cancer by
almost 50% in all age groups. In the subset of patients with ductal atypia,
prophylactic tamoxifen reduced breast-cancer incidence by 86%.7
Established algorithms also exist for guiding case management of women
identified with higher breast-cancer risk.8
Despite the availability of these preventive
approaches, traditional means of assessing breast-cancer risk are not very
useful in identifying patients who could benefit from them. Between 50% and
70% of women who develop breast cancer have no identifiable risk factors,
other than age, using today’s standard risk-assessment tools. Statistical
risk-assessment methods — such as the Gail model, which uses a woman’s
personal and family medical history to estimate risk — have utility more as
an epidemiological tool than for identifying personalized risk.9
For example, the Gail model is known to underestimate risk in some patient
populations and often overestimates risk in others.10 Because
these traditional methods have proved inadequate, there has been an ongoing
interest in developing readily accessible biomarkers that can identify
breast-cancer risk with an acceptable degree of individual predictive
accuracy. These biomarkers should ideally be present in a reasonable number
of at-risk individuals, minimally invasive, and reversible with prevention
interventions. A number of risk biomarkers have been identified (e.g.,
mammographic breast density, serum insulin-like growth factor-I and its
binding protein, insulin-like growth-factor-binding protein, serum levels of
estradiol and testosterone in postmenopausal women, and breast-tissue
markers).
Among those potential tissue biomarkers, findings of
epithelial atypia have long held tremendous promise. Numerous prospective
studies involving more than 20,000 women have shown ductal atypia, either
cytologic or histologic, to be an effective predictor of breast-cancer risk
in individuals.1,2,11-13 Until recently, a clinical model in
which benign breast tissue can be easily obtained and repeated over time for
risk assessment has remained elusive. Open biopsies of palpable masses or
mammographic abnormalities over time is impractical because the lesion of
interest is removed on the first biopsy sample. Repeated random open
biopsies for risk surveillance are more logical, but few women would subject
themselves to such a procedure, especially over many years, and such
biopsies may contain little of the terminal ductal-lobular units unless they
were specifically directed. Random periareolar FNA is simple and
inexpensive, and may be repeated with minimum morbidity. The majority of FNA
samples have been noted to be cellular in previously identified high-risk
women. The cytologic identification of proliferative breast disease with
atypia in these specimens has been associated with future cancer
development. Finally, atypia in NAF has been shown to have a prospective
association with increased breast-cancer risk. Concerns in the past,
however, were that these samples may be acellular and that not all women
readily produce fluid.
The reason cytology sampling has not been used more
widely until recently has to do with the difficulty of collecting breast
samples. There are now emerging newer, more acceptable collection methods
that overcome these difficulties. Before describing these technologies and
alternative methods, it is helpful to examine the rationale for this tissue
biomarker in more detail.
Validation of breast epithelial atypia biomarker
Our current understanding of cytomorphology of breast
specimens was first reported more than 50 years ago. Dr. George Papanicolaou
and colleagues published their research with exfoliative cytology samples of
the breast in 1958.14 Dr. Papanicolaou carefully illustrated the
cellular composition of both nipple aspirates and spontaneous nipple
discharges in asymptomatic and symptomatic women. Dr. Papanicolaou proposed
a five-tiered classification system for these cytologic findings. He
concluded that, despite its limitations, cytologic examination should be
performed in every case of spontaneous discharge. He suggested that the
Papanicolaou technique could be used to detect carcinoma at an earlier,
pre-clinical stage. This could be incorporated into the physical examination
with little loss of time and without danger to the patient.
The logic of Papanicolaou’s observations stemmed from
breast anatomy and what is known about the genesis of breast cancer. Ductal
epithelial cells compose the lining of the roughly 12 ducts that end in a
woman’s nipple. These ducts are part of a branching series of ductal-lobular
units within the breast. It is believed that most invasive breast cancers
begin from pre-cancerous lesions in these epithelial cells. These changes
are now widely recognized to have a closer biologic association to elevated
risk of developing breast cancer than other risk biomarkers.
The reliability of cytologic atypia as a
breast-cancer risk biomarker is well established in the literature, starting
with a major prospective study. In that research, Wrensch and Petrakis, et
al, of the University of California-San Francisco studied 2,701 women
followed for an average of 12.7 years and showed that asymptomatic women
with atypia in NAF had a breast-cancer risk 4.9 times greater than women who
did not yield NAF.11
In 2001, a follow-up study confirming the original work was published based
on following 7,600 women for 21 years.12
Three other studies found similar relative risks (RR)
for women with atypia discovered through open biopsy or FNA:
- DuPont and Page (NEJM, 1985) found 5.3x RR with biopsy-proven
atypia in 3,303 women
followed for an average of 17 years.13 - Fabian (JNCI, 2000) found 5x RR with atypia discovered by FNA
in 480 women followed for four
years.2 - Hartmann (NEJM, 2005) found 4.2x RR with biopsy-proven atypia
in 9,087 women followed an
average of 15 years. Women under 45
had a 6.99x RR.1 - Two recent studies looked at risk associated with the presence of
epithelial cells in NAF,
regardless of whether the cells were
normal or atypical: - Buehring, et al (Epidemiology, 2006) found 1.92x RR in 972
women followed for 25 years.15 - Baltzell (2008) found a 1.9x RR in 946 women followed for 20.7
years.16
The National Cancer Institute, the American Cancer
Society, and the American Society of Breast Surgeons17 have all
recognized cytologic atypia as an objective, valuable biomarker of
breast-cancer risk.
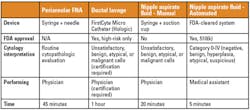
State of the technology for breast-fluid collection
Mammary samples can be collected either invasively or
non-invasively. Non-invasive methods include a manual-extraction approach
and newer automated system, which appears to address problems associated
with the manual method. The manual-collection method begins with the
application of heat and massage to the breast, followed by use of a suction
cup and attached syringe, or, in some cases, a breast pump. The complexity
of the procedure is such that a physician is often required to perform it.
The method can also be time consuming. For these reasons, providers often do
not consider it practical, and its use is mainly in the research setting.
Next, an automated instrument uses the successive
application of heat, massage, and suction, but a machine similar to a breast
pump performs the cycle automatically. Adjustable breast cups fitted with
disposable sample-collection cups are placed on both breasts simultaneously
and are then adjusted around the nipple and areola. No topical anesthetic is
needed beforehand. Because the procedure is automated, it takes only five
minutes and can be performed by an assistant rather than a physician. These
qualities make it more suitable for settings such as primary-care or OB-GYN
offices. The system has undergone 510(k) clearance by the U.S. Food and Drug
Administration (FDA). The approval states that “The collected fluid can be
used in the determination and/or differentiation of normal versus
premalignant verses malignant cells.” A published prospective observational
clinical trial found that the device was well tolerated, safe, and
effective.18 Proctor, et al, demonstrated that results from NAF
collected via the automated system are stratified equivalently to NAF
samples collected manually (i.e., have the same percentage of non-yielders,
acellular samples, atypia, and so on).
Nipple fluid can be obtained from many women, with
reports of NAF production ranging from 25% to more than 95% of asymptomatic
women.18 Breast tissue and ductal systems are influenced by
hormones, which affect the ability to produce NAF. Other intrinsic breast
characteristics can influence the ability to obtain NAF. On average, about
50% of women produce fluid. Women who do not produce are termed
“non-yielders.” They have the lowest risk of developing breast cancer. Those
that do produce NAF commonly have an acellular specimen; that is, no
epithelial cells were exfoliated with the fluid. This is a normal result
indicating that the woman’s risk is only slightly higher than someone who
does not produce fluid. The epithelial cells lining the breast ducts are
constantly producing and reabsorbing NAF at similar rates, so often there is
no accumulation of NAF to collect. Slight changes in the ducts can cause the
cells to produce more NAF than they can absorb, resulting in a slight
accumulation. Again, studies have shown that these women (i.e.,
“non-yielders”) have a statistically decreased breast-cancer risk compared
to women who do yield NAF.11,12
Invasive approaches to collecting specimens include
random periareolar FNA and ductal lavage (DL). Some of the earliest
descriptions of the FNA technique were published by Drs. Ward and Marshall
in a cohort of high-risk women from the University of Utah in 1990.19
After the breast skin is numbed with a local anesthetic, the needle is
inserted into each quadrant of the breast, approximately one centimeter (1
cm) from the areola. The needle is then repositioned eight to 10 more times
in all areas within the quadrant. The procedure is generally performed by an
interventional radiologist or cytopathologist, making it impractical for
wide clinical use.
Like FNA, DL is not considered a practical option for
risk screening of the type discussed herein, for multiple reasons. Most
importantly, its FDA clearance limits it use to women already known to be at
high risk.20 In other words, it is not indicated for women who
have no known risk factors, a cohort that constitutes 50% to 70% of women
who will develop breast cancer, the group most in need of risk assessment.
Another limitation of DL is that clinicians must complete a
specialty-training program to perform the procedure. The procedure involves
the insertion of a small catheter into the ducts emerging from the nipple,
flushing with saline, and subsequent collection of the flushed saline
containing any ductal cells. Pathologists must also be certified to evaluate
and report results because evaluation involves precise differentiation
between different grades of atypia.
In contrast, cytological examination of NAF does not
require special certification because the results are used for risk
stratification only, not pathologic diagnosis. Results are reported in just
five categories focused on the presence or absence of atypia with no need to
determine grades of atypia. Therefore, most laboratories with routine
cytology capability can evaluate and report NAF status after brief training.
Spontaneous nipple secretions or discharges
Spontaneously secreted or discharged fluid is
different from NAF, both for purposes of pathologic evaluation and in the
clinical origins. Spontaneously secreted or discharged fluid is a symptom,
whereas the NAF discussed herein is collected from asymptomatic women.
Although the causes are not well understood, spontaneous secretions can be
symptoms of conditions ranging from mastitis and hormonal imbalances to
tumors. Most likely, they occur because of endocrine-related changes or use
of particular medications.
It must be emphasized that a cytologic evaluation
does not in any way replace mammograms, manual exam, or other diagnostic
tests. Nipple aspirate fluid cytology results are not diagnostic. Breast NAF
is a means of risk assessment, and its routine clinical use should be
confined to asymptomatic women with a normal breast examination. Although
breast NAF cytology was designed to detect cytologic changes associated with
benign breast lesions, there will certainly be breast cancers which cannot
be detected by NAF. In the absence of information on sensitivity and
specificity for detection of carcinoma, NAF should be considered a method of
risk assessment only, and this must be emphasized to women undergoing the
procedure to ensure that a benign NAF does not result in false reassurance,
causing a woman to ignore symptoms of breast cancer or neglect screening
tests of proven value such as mammography.
Best practice: specimen acquisition, transport, and labeling
The discussion from this point forward assumes the
use of an automated system for collecting NAF because of its practical
advantages over other methods. Collection will most likely take place in a
primary-care or OB-GYN office or at a breast center. NAF evaluation is
particularly appropriate for asymptomatic, pre-menopausal women ages 25 to
55 — the same group for whom cervical-cancer screens are recommended. Much
of the rationale for this age range, including breast density and the
virulence of cancer in younger women, has already been described. Another
relevant factor is that post-menopausal women tend to produce less NAF.
There is no specific cutoff age at which the test is suddenly not useful.
Post-menopausal women can continue to benefit from NAF evaluation as long as
they produce NAF, though the test is less meaningful for older women who are
non-yielders. Also, NAF evaluation can begin earlier than 25 for patients
with other known risk factors. As with cervical Pap tests, breast-health
screening would ideally be recommended as part of a woman’s regular health
check-up.
Specific directions for handling samples prior to
submission to the laboratory are as follows: Samples are collected with a
custom, non-cell-binding swab that is supplied as part of the collection kit
for the automated device. The swab is placed directly into a non-gyn
liquid-based cytology (LBC) fixative vial for transport to a pathology lab.
Clinicians have the option of submitting separate
vials for each breast’s sample, or submitting the samples together in a
single vial. The argument for submitting separate vials is that if an actual
malignancy is revealed, the clinician will want to know which breast is
affected. Absent this possibility, there is no advantage for risk-assessment
purposes to submitting separate vials. A woman with atypia in one breast has
a nearly equal chance of developing breast cancer in either breast due to
the “field effect” whereby both breasts together constitute a single organ.
Because of the diagnostic circumstance just mentioned, labels should
indicate whether a patient’s two samples are pooled or placed in different
vials.
Best practice: slide preparation and staining
It is preferable to prepare NAF slides with
liquid-based cytology, or LBC, technique, using cellular concentration and
monolayer slide method. This approach aids interpretation, because it
optimizes cellularity. While cytocentrifugation yields acceptable slides, a
portion of the sample might not be deposited onto the slide, so some cells
may be lost with this method. Cell block preparation should be avoided with
NAF samples because the samples are normally hypocellular. All
breast-cytology slides should be stained with the Papanicolaou stain.
Best practice: slide screening and interpretation
Macrophages (foam cells), squamous cells (nipple
contaminate), ductal epithelial cells, and less frequently, inflammatory
cells and blood are among the cell types pathologists will observe in NAF
samples. Only a small percentage of NAF samples from asymptomatic women —
from 0.7% to 2.7%, according to studies — are likely to display atypia.
“Abnormal” samples are those that display atypia, suspicious cells, or
malignant cells. These samples are somewhat easier to differentiate than
“normal” samples, which include those with acellular, normal epithelium, or
hyperplasia. In normal, asymptomatic patients, NAF is generally acellular or
hypocellular.
The pathologist’s role in interpretation of NAF,
especially from automated collections, is very different than when
interpreting diagnostic samples, such as those obtained from FNA or open
biopsy. A diagnostic test is defined as a specific test used to confirm the
presence of disease. In the case of breast cancer, once an abnormality has
been identified through screening, diagnostic testing typically includes a
biopsy for direct tissue examination and determination of malignancy. An
automated system-collected sample is used for risk assessment purposes only,
so no clinical impressions will accompany the sample, nor will any treatment
course be based directly on the finding. Screening is generally defined as
systematic testing for the early detection of cancer in people with no
symptoms of the disease. Screening tests are not performed to diagnose a
disease but to identify currently asymptomatic individuals for whom more
specific diagnostic testing is warranted. In developing cancer-screening
summaries, the National Cancer Institute (NCI) PDQ Screening and Prevention
Editorial Board uses the following definitions:
- 1. Screening is a means of detecting disease early in asymptomatic
people. - 2. Positive results of examinations, tests, or procedures used in
screening are usually not
diagnostic but identify persons
at increased risk for the presence of cancer who warrant
further evaluation.
Reporting specimen adequacy with NAF results
Early work on nipple-fluid cytology by Drs.
Papanicolaou14
and King21 and, later, by Procter18 had proposed
classification schemes in which the first category, Category 0, was an
inadequate or non-diagnostic sample. In the newest classification scheme
described below, for breast-cancer risk assessment only, Category 0 instead
indicates a meaningful result. This is because of an evolution in thinking
about hypocellular and acellular NAF samples.
It is now recognized that hypocellular samples are
normal, and many women will produce samples that are acellular. In addition,
many women will produce no NAF at all. None of these circumstances are
considered “unsatisfactory,” “inadequate,” or “non-diagnostic” today. Normal
findings indicate only a slightly elevated cancer risk over women who
produce no NAF at all. Only abnormal findings (atypia or suspicious for
malignancy) indicate a significantly increased risk.
There is no expectation that women with healthy
glands or ducts will produce fluid or, if they do, that epithelial cells
will be present in the sample. Thus, it is not considered mandatory for the
pathologist to provide a specimen-adequacy statement in the situations just
described.
There is still an important quality-assurance role
for adequacy statements when they might convey useful information about a
lab’s NAF-processing quality. A lab that consistently produces “blank”
slides may be doing so because samples are being collected or processed
improperly. In such a case, management should be notified so the situation
can be researched and addressed.
Recommended classification system for reporting NAF results
There are several reasons for reporting NAF results
via a standardized classification system:
- 1) Results can be communicated clearly and unambiguously. 2) Physicians often want their staffs to alert them to any results
- 3) Classification categories reduce complication in physician
reports. - 4) A classification system helps labs and other healthcare providers
easily compile statistical
reports, electronically or
otherwise. - Hartmann LC, et al. Benign breast disease and the risk of breast
cancer. NEMJ. 2005;353(3):229-237. - Fabian CJ, et al. Short-term breast cancer prediction by random
periareolar fine-needle aspiration cytology and the Gail risk model.
J Natl Cancer Inst. 2000;92(15):1217-1227. - Masood S. Cytomorphology as a risk predictor: experience with fine
needle aspiration biopsy, nipple fluid aspiration, and ductal lavage.
Clin Lab Med. 2005. 25(4):827-43, viii-ix. - Fabian CJ, Kimler BF. Breast cancer risk prediction: should nipple
aspiration fluid cytology be incorporated into clinical practice? J
Natl Cancer Inst. 2001;93(23):1762-1763. - West JG, Hollingsworth AB. Screening for breast cancer risk in the
obstetric/gynecological setting: a breast surgeon’s perspective.
Expert Rev Obstet Gynecol. 2008;3(1):59-63. - American Cancer Society. Estimated New Cancer Cases and Deaths by
Sex, US, 2008. Available from
http://www.cancer.org/downloads/stt/CFF2008Table_pg4.pdf .
Accessed December 15, 2008. - Fisher B, et al. Tamoxifen for prevention of breast cancer: report
of the National Surgical Adjuvant Breast and Bowel Project P-1 Study.
J Natl Cancer Inst. 1998;90(18):1371-1388. - Hollingsworth AB, et al. Current comprehensive assessment and
management of women at increased risk for breast cancer.
Am J Surg. 2004;187(3):349-362. - Gail MH, et al. Projecting individualized probabilities of
developing breast cancer for white females who are being examined
annually. J Natl Cancer Inst.1989;81(24):1879-1886. - Pankratz VS, et al. Assessment of the accuracy of the Gail model in
women with atypical hyperplasia. J Clin Oncol.
2008;26(33):5374-5379. - Wrensch MR, et al. Breast cancer incidence in women with abnormal
cytology in nipple aspirates of breast fluid. Am J Epidemiol.
1992;135(2):130-141. - Wrensch MR, et al. Breast cancer risk in women with abnormal
cytology in nipple aspirates of breast fluid. J Natl Cancer Inst.
2001;93(23):1791-1798. - Dupont WD, Page DL. Risk factors for breast cancer in women with
proliferative breast disease. NEJM. 1985;312(3):146-151. - Papanicolaou GN, et al. Exioliative cytology of the human mammary
gland and its value in the diagnosis of cancer and other diseases of the
breast. Cancer. 1958;11(2):377-409. - Buehring GC, et al. Presence of epithelial cells in nipple aspirate
fluid is associated with subsequent breast cancer: a 25-year prospective
study. Breast Cancer Res Treat. 2006;98(1):63-70. - Baltzell KA, et al. Epithelial cells in nipple aspirate fluid and
subsequent breast cancer risk: a historic prospective study. BMC
Cancer. 2008;8:75. - Am Society of Breast Surgeons Ductal Cell-Based Risk Assessment.
2007. Available from
http://breastsurgeons.org/officialstmts/Ductal_Cell.pdf .
Accessed December 5, 2008. - Proctor KA, Rowe LR, Bentz JS. Cytologic features of nipple aspirate
fluid using an automated non-invasive collection device: a prospective
observational study. BMC Womens Health. 2005;5:10. - Ward JH, et al. Detection of proliferative breast disease by
four-quadrant, fine-needle aspiration. J Natl Cancer Inst.
1990;82(11):964-966. - Dooley WC, et al. Ductal lavage for detection of cellular atypia in
women at high risk for breast cancer. J Natl Cancer Inst.
2001;93(21):1624-1632. - King EB, et al. Nipple aspirate cytology for the study of breast
cancer precursors. J Natl Cancer Inst. 1983;71(6):1115-1121.
requiring their immediate
attention. A NAF- classification
system can aid this process by concisely and clearly
results.
Conclusion
Pathologists can play a key role in understanding the
genetic and metabolic differences unique to the individual patient. This
knowledge could then be used to tailor a custom program of prevention that
offers maximum potential and minimizes possible side effects. These findings
do not take the place of standardized screening programs but can supplement
the current personal and family history for a given patient. Much progress
has been made in identifying treatments and lifestyles for prevention of
breast cancer; but until recently, there was no practical, effective means
of risk assessment to identify appropriate candidates for these approaches.
The majority of women who develop breast cancer have
no identifiable risk factors. A finding of epithelial atypia, either by
histology or cytology, has long been recognized as an important
breast-cancer risk biomarker; but, to date, there has not been a practical
way to apply this knowledge to the general population as a screen. With NAF
cytology, we might have the opportunity to identify and refer high-risk
women for enhanced surveillance programs earlier, and offer a woman and her
physician the chance to make more informed decisions about minimizing her
risk of breast cancer.
Joel Bentz, MD, is clinical
professor of pathology at the University of Utah in Salt Lake City. He is a
member of the Scientific Advisory Board of NeoMatrix LLC, in Irvine, CA,
which manufactures the HALO system.
References
MLO’s
Continuing Education Test is available online only.
Print out and mail a copy with your check, or use the new online CE test
and convenient online payment feature available through the auspices of
Northern Illinois University.
Go to www.mlo-online.com and
look under CE Tests.