The 2023 Medical Laboratory Observer (MLO) State of the Industry (SOI) survey on Disease Management focused on testing for respiratory infections and sexually transmitted infections (STI). Alongside the quantitative survey results, we present insights from U.S. lab professionals and lab equipment suppliers on the trends they are seeing in these two disease management areas.
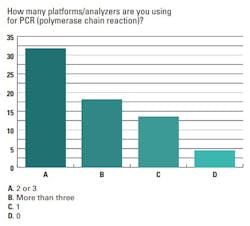
When asked how many platforms/analyzers their lab uses for polymerase chain reaction (PCR) testing, 64% of respondents said two-to-three PCR platforms/analyzers, 18% said more than three and 14% said they use only one.
Tamara Ranalli, Senior Vice President of Molecular Business Unit, QuidelOrtho, commented on PCR trends:
“Laboratory quality molecular testing solutions, especially those using reverse transcriptase-polymerase chain reaction (RT-PCR), continue to move closer to the patient while becoming easier to use and with time to results rivaling those of rapid antigen tests. More syndromic tests are likely to emerge, reflecting the need to test numerous pathogens quickly while reducing technician hands-on time and supporting antibiotic stewardship. In addition, ease of use, even within a laboratory environment, is a desired feature given the reduction in available molecular biologists in labs.”
“As SARS-CoV-2 volumes decreased, labs have been looking for ways to take their new PCR capacity and use it as an opportunity to offer other tests, such as onboarding tests for sexually transmitted infections or monitoring of viral infections in transplant patients,” said Alesia McKeown, Ph.D., Scientific Partner, Roche Diagnostics. “That shift can help labs avoid sending tests out, saving expenses and decreasing the time to get results in the hands of clinicians and patients.”
The 2022–2023 respiratory season
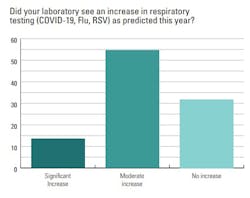
Lab professionals were asked if they had seen an increase in respiratory testing (COVID-19, flu, RSV) as predicted this year. Most reported an increase, with 14% seeing a significant rise in tests and 55% a moderate uptick. About one-third of respondents (32%) said they experienced no increase in respiratory testing.
Lee Panton, former Lab Director from California who now serves as a consultant, said she has seen an increase in requests for complete panels for respiratory virus screening, but the lab for which she consults does not have molecular testing capability. She described how they handle these test requests:
“In each case, we need to contact the ordering MD and ask for a list of the individual tests that are needed. Then we do the influenza, RSV and COVID tests in house with antigen kits. Other tests are sent to an outside reference lab. This does not allow for prompt diagnosis of all potential respiratory infections. The volume of panel testing is still low. Our corporate office must approve any new testing systems that each of our hospitals are interested in acquiring. Currently, we do not have approval to bring in the new molecular panels.”
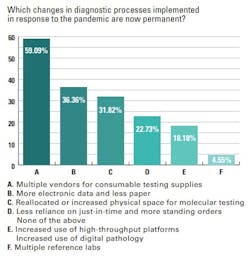
The survey found many changes in diagnostic processes for respiratory testing that were implemented in response to the pandemic are now permanent. Topping the list was multiple vendors for consumable testing supplies (59%), followed by more electronic data and less paper (36%), and reallocated or increased physical space for molecular testing (32%).
“As labs continue to struggle with increased post-pandemic testing volumes and decreased staffing, automation is more important than ever to help improve access to testing, accuracy of testing, and speed of testing,” Nikos Pavlidis, VP/GM Diagnostics, BD Life Sciences, commented. “High-throughput, fully integrated preanalytical and analytical systems can enhance both laboratory operations and patient management around STI testing by processing tests at a faster rate and at a higher volume than a lab might previously have experienced."
Nearly a quarter of survey respondents (23%) said they continue to have less reliance on just-in-time delivery of products and instead have more standing orders in place with suppliers. Increased use of high-throughput platforms and digital pathology remain in place in 18% of labs surveyed, and 5% say they are still using multiple reference labs.STI testing trends
U.S. medical lab professionals surveyed reported an increase in STI testing, but mostly modest, with 41% reporting a moderate increase in testing in this area, and 5% a significant increase. Over half of survey respondents (55%) reported no increase in STI testing over the past 12 months.
“There has been an increase in the number of reported cases of STIs, which could be due to factors like access to care and the willingness of professionals to talk to patients about the importance of screening when asymptomatic,” said Dr. Damian Alagia III, MD, MS, MBA, FACS, FACOG, Senior Medical Director for Advanced Diagnostics and Women’s Health, Quest Diagnostics. “It seems the more discussion there is about the impact STIs can have on fertility, quality of life, and a person’s health and well-being, the more concerned people are. Yet, STIs are an area of healthcare with significant gaps in care relative to guidelines.”
Patient hesitancy to pursue education on STIs and testing was called out as a challenge by various experts interviewed.
Pavlidis commented on a 2023 BD commissioned Harris Poll survey that found women feel less knowledgeable about STI testing and treatment options compared to other vaginal diseases and infections:1
“These findings show the importance of providing patients with the knowledge they need to address and prevent STIs. With the CDC reporting that STIs make up five of the top 10 reportable diseases in the U.S.—with increasing incidence—knowledge and education are critical for patients, as is the development of targeted point-of-care diagnostic technologies.”STIs by the numbers
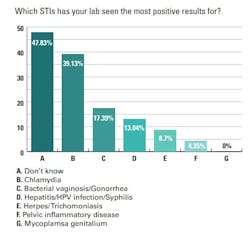
Looking at testing for specific STIs, chlamydia was highest on the list, with 39% of lab professionals seeing the most positive test results for this infection among their patient populations. Labs reported a 17% increase in positive results for bacterial vaginosis (BV) and gonorrhea, and a 13% increase for hepatitis, syphilis, and HPV infection.
Lower on the list were herpes and trichomoniasis, with a 9% increase in positive results for both STIs reported, and pelvic inflammatory disease, of which 4% of lab professionals said they have seen increased positive results.
Panton said recent false positive rates for rapid plasma reagin (RPR) tests have presented a major challenge to her lab’s STI testing, especially with the increase in confirmed syphilis cases in her area. She stated:
“We send out RPR testing to a reference lab and results are taking longer than expected. A big issue that started about 9 months ago is ‘false’ positive RPR tests. We get the RPR as 1:1 dilution positive. The confirming FTA is negative. This is happening mostly on pregnant women who are in for their pre-delivery tests. It is causing a lot of confusion because we must contact the MD and report it to Public Health before the FTA is completed.”
STI supply testing challenges
With some STIs on the rise, testing supply shortages continue to pose challenges. Among those surveyed, 55% said they have had trouble acquiring swabs for testing, 36% blood specimen tubes, 27% urine collection devices, and 18% assays.
Advice for labs in disease management testing in 2023 and beyond
Respiratory testing
When it comes to respiratory testing trends, a pervasive message to labs is — prepare for the unexpected.
“Seasonal irregularities do not allow for good predictive models and given supply chain pressures for some items that may not have fully abated, labs may be challenged in maintaining adequate inventories for the unexpected. However, a combination of multiplex assays and a variety of platforms offering throughput flexibility can help cover the bases when used appropriately,” said Ranalli.
“Labs have to be ready to pivot quickly so they can address the escalation of any type of respiratory virus, as we’ve seen how quickly local transmission of viruses can move to larger public health issues,” said McKeown. “Part of this ability to respond involves having the right tools, such as PCR instruments, which many labs brought on or expanded capacity during the pandemic.”
STI testing
Experts emphasized the need for closer partnerships between labs and healthcare providers to support increased STI education and testing.
McMullen encouraged labs to partner with healthcare practitioners to help meet their testing needs, and share information on current and new sexual health test formats available, such as point-of-care testing.
Dr. Alagia stressed the importance of understanding that STI testing is not just a test a provider is performing, but rather part of a bigger picture where the provider is engaging in patient care, with results impacting a person’s psychological and sexual health and wellbeing.
“It’s our position that providing a test doesn’t stand alone but needs to be connected to a system of patient care,” said Dr. Alagia. “Those who test for STIs are providing a piece of the clinical care pathway that impacts a patient and their family, and need to ensure that testing is high-quality, consistent, accessible, reliable, and closely monitored. Testing providers must recognize they are essential to patient management; a physician can't diagnose, counsel, or treat without first receiving a test result.”
REFERENCES
1. New Harris poll finds women want more education on sexually transmitted infections testing and treatment. BD Newsroom. Accessed June 26, 2023. https://news.bd.com/2023-04-19-New-Harris-Poll-Finds-Women-Want-More-Education-on-Sexually-Transmitted-Infections-Testing-and-Treatment.