Unlocking the potential of ctDNA-based MRD testing to improve cancer management
Over the past 20 years, clinical oncology practice has evolved in several important ways. Most excitingly, treatment strategies have become more personalized to the individual patient. This level of personalization is often only possible by implementing appropriate diagnostic tests to measure particular characteristics of an individual patient’s cancer.
One of the fastest evolving and most exciting areas of personalized oncology care is minimal residual disease (MRD) testing for solid tumor cancers. In simple terms, MRD refers to cancer that may persist in a patient following curative-intent treatment, such as surgery. This residual cancer, or MRD, may be attributed to a microscopic deposit of cancer cells that is not yet large enough to be visualized by advanced radiographic tools. Left unchecked, these cells can lead to cancer recurrence or relapse that is eventually revealed by radiography (e.g., CT or PET scans).
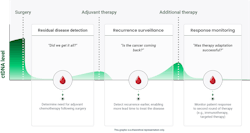
MRD testing is often initiated after surgical resection to detect any residual disease that may persist and is otherwise unable to be visualized. Results in this setting may aid the oncologist to evaluate the suitability of additional treatments, such as chemotherapy.
Following treatment, MRD testing can be performed for several years afterward to monitor for cancer relapse and recurrence. MRD testing has been consistently shown to reveal recurrent cancer in advance of conventional approaches such as protein biomarker assessment and imaging techniques, which lack the resolution for identifying minute quantities of tumor and/or require a visit to the hospital for a radiology appointment. In certain settings, some patients neglect to come back to the hospital for routine surveillance testing with imaging. MRD testing, however, is much more convenient as it is performed with a simple blood draw that can be managed by mobile phlebotomy. This enables seamless access for the patient to important diagnostic testing.
In colon cancer, for example, as many as 30–40% of patients can recur after surgery with or without chemotherapy.2 Recurrence monitoring for colon cancer depends on the cancer stage and other factors, but involves periodic imaging tests, including computed tomography (CT) scans, magnetic resonance imaging (MRI) and colonoscopies, as well carcinoembryonic antigen (CEA) blood tests.
These conventional approaches have significant limitations. CEA testing identifies CRC recurrence in only about 59% of cases.3 Due to the poor sensitivity and specificity of CEA and limited resolution of imaging, these methods can miss cancer in early stages, when treatment has the greatest potential to eradicate disease.
ctDNA indicates the presence of MRD
In patients with a solid tumor cancer, such as colon or breast cancer, DNA molecules from the tumor are shed into the blood stream. These molecules can be detected using a so-called “liquid biopsy” test — in other words, a noninvasive test intended to identify tumor-derived material in bodily fluids, most commonly plasma derived from whole blood.
While normal cells also shed their DNA into the blood, circulating tumor DNA (ctDNA) is distinguished from normal cell-free DNA by the presence of cancer-specific mutations. The detection of ctDNA after treatment indicates that disease is still present and signifies a high risk of cancer recurrence in the absence of additional treatment. Because of the unique specificity of ctDNA for an individual cancer, a ctDNA-based MRD test with adequate sensitivity can enable identification of residual or recurrent cancer earlier than ever before (See Figure 1).
MRD testing is used to help answer the questions, “Did surgery remove all of my cancer,” and “Did my cancer return”? MRD testing can also be used to help monitor how a patient is responding to treatment, as indicated by changes in ctDNA levels during treatment. When used for treatment monitoring, MRD tests can help indicate when therapy is working and provide confidence that disease has been successfully treated. In contrast, increasing ctDNA levels may indicate a patient’s disease is not responding to the prescribed treatment and other interventions, including clinical trials, and should be explored.
Several clinical studies have shown that ctDNA can be an early marker of solid tumor cancer disease recurrence. For instance, one study found that individuals with negative ctDNA levels remained recurrence-free while 77% of patients with positive ctDNA levels relapsed during a median follow-up of 49 months. Notably, positive ctDNA preceded radiologic and clinical evidence of recurrence by a median of 3 months.4
While this and other data point to the significant potential of ctDNA-based MRD tests to aid clinical care, challenges remain. One crucial challenge with ctDNA analysis is the detection of extremely low numbers of tumor DNA molecules that may be present following treatment, which requires a highly sensitive testing technology for reliable detection.5
How a highly sensitive test can change the game
Haystack Oncology has developed a highly sensitive ctDNA-based MRD test to detect residual or recurrent cancer to better inform clinical decisions. Haystack MRD is a personalized ctDNA detection test that has been developed based on over two decades of landmark technology and clinical advances in the fields of cancer genomics and liquid biopsy led by Dr. Bert Vogelstein and his team at Johns Hopkins University. This team discovered the genetic basis of cancer, sequenced the first cancer exomes, and developed the first liquid biopsy techniques, laying the groundwork for the current era of personalized oncology and targeted therapies. The Haystack technology was developed specifically to detect ultralow levels of ctDNA typically seen in early-stage patients. Haystack MRD is tumor-informed, which means the test is custom-designed for each patient based on sequencing the patient’s tumor to identify up to 50 different variants in the patient’s ctDNA. This contrasts with tumor-agnostic methods, which use a single test to look for a fixed number of mutations in the blood without the benefit of personalization to the patient’s tumor. This lack of personalization of tumor-agnostic tests often identifies only a couple of variants in ctDNA, which is directly related to limitations in sensitivity yielding high false-negative rates and inconsistent results in clinical evaluations.
Another challenge of MRD testing is distinguishing tumor-derived DNA molecules from the normal (non-tumor) DNA molecules that are released into circulation from normal cells. For every molecule of ctDNA, there may be a million or more normal DNA molecules that obscure the cancer-specific signal. Therefore, a sensitive MRD test needs to be able to remove the background noise of normal DNA to detect those precious few ctDNA molecules in a tube of blood. As stated earlier, Haystack’s technology was designed specifically to detect these low-abundant ctDNA molecules, and so every aspect of the test, from the chemistry and workflow to the bioinformatics, has been purpose-built to reduce background noise and detect ctDNA.
With more Americans than ever before now being touched by cancer, detecting residual or recurrent disease and identifying better treatment options is essential to increasing survival rates and improving quality of life. Additionally, cancer patients experience both a clinical and economic burden, with treatment imposing side effects, requiring significant amounts of time and money and causing strain on personal relationships. This underscores the importance of identifying cancer as soon as possible and ensuring that the right treatment goes to the right patient at the right time.
ctDNA use in clinical trials
One of the most exciting opportunities for MRD testing is to support clinical research, including the development of novel therapies. MRD testing can be leveraged for patients who have the highest risk of recurrence and therefore are more likely to benefit from therapy. Further, MRD testing can provide a real-time indication of patients’ disease response to treatment.
ctDNA testing to help reduce adjuvant therapy
MRD testing has significant potential to guide treatment decisions following curative-intent surgery. The presence of post-surgical ctDNA indicates that surgery was not successful in the total eradication of disease. Across several studies, ctDNA has been shown to be predictive of cancer recurrence, suggesting a role to better identify patients appropriate for adjuvant treatment.
Adjuvant treatment typically refers to chemotherapy, alone or with other treatments, such as radiation, following surgical resection — removal of a solid cancer tumor. This treatment essentially seeks to mop up any residual cancer cells that can remain in the body following surgery. However, many patients are successfully treated with surgery alone and do not need to undergo costly systemic therapy that is often fraught with side effects, from fatigue and nausea to neuropathy. The challenge in clinical practice is identifying those patients whose tumors have been completely surgically treated and can therefore avoid adjuvant therapy. MRD testing with an appropriate test has demonstrated an important role in this decision process.
The seminal DYNAMIC study suggests post-surgical MRD testing can dramatically improve the identification of patients for adjuvant therapy. Published in the New England Journal of Medicine in June 2022, DYNAMIC is the first completed randomized, interventional study to investigate the clinical utility of MRD testing to help guide the use of chemotherapy after surgical resection. The MRD test used in the study was based on a precursor to the current Haystack MRD technology. Among 455 stage II colon cancer patients, individuals with positive ctDNA results post-surgery were given adjuvant chemotherapy, whereas patients with negative ctDNA results did not receive chemotherapy. In contrast, patients in a group representing the standard of care received adjuvant chemotherapy based on traditional risk assessment using clinicopathological features such as tumor size and lymph node involvement. Results showed chemotherapy usage was halved in the ctDNA-guided group versus the standard of care group (with 15% of patients receiving therapy versus 28%, respectively) with no impact to two-year recurrence-free survival rates.6 This is the first study to demonstrate the clinical utility of using MRD testing to help guide the use of chemotherapy in cancer patient after surgery, providing strong evidence that ctDNA testing can help identify patients who are less likely to benefit from chemotherapy.
ctDNA-based tumor informed MRD testing has emerged as a mainstream cancer diagnostic
MRD testing is a fast-growing diagnostic approach that is expected to become an integral part of personalized cancer care. The ability to detect MRD can help physicians make better-informed decisions about their patients’ treatment. Per the current standard of care, many patients who are treated with surgery alone receive unnecessary adjuvant therapy and suffer associated toxicities and costs with minimal therapeutic benefit. The DYNAMIC trial showed that MRD testing can help identify patients that are more likely to benefit from adjuvant therapy by identifying the presence of ctDNA in their blood – an amount that may be indiscernible by traditional clinical or imaging assessments, yet still represents the presence of residual cancer. Additional research is underway across various cancer types and early evidence suggests MRD testing’s potential to reduce adjuvant therapy in other cancers beyond stage II colon cancer.
One thing is certain: Uncovering insights into residual or recurrent cancer is vital to helping patients overcome this deadly disease. It is essential for oncologists and laboratorians to stay abreast of innovations and developments like ctDNA-based MRD testing to ensure all cancer patients receive the most advanced care for optimal outcomes.
References
1. Colorectal cancer statistics. Cancer.org. Accessed May 23, 2024. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html.
2. Cafasso J. Colon cancer recurrence: Rates, treatment, and prevention. Healthline. Published September 14, 2022. Accessed May 23, 2024. https://www.healthline.com/health/colorectal-cancer/colon-cancer-recurrence.
3. Liemburg GB, Brandenbarg D, Berger MY, et al. Diagnostic accuracy of follow-up tests for detecting colorectal cancer recurrences in primary care: A systematic review and meta-analysis. Eur J Cancer Care (Engl). 2021;30(5):e13432. doi:10.1111/ecc.13432.
4. Wang Y, Li L, Cohen JD, Kinde I, et al. Prognostic Potential of Circulating Tumor DNA Measurement in Postoperative Surveillance of Nonmetastatic Colorectal Cancer. JAMA Oncol. 2019;1;5(8):1118-1123. doi:10.1001/jamaoncol.2019.0512.
5. Galot R, Machiels JH. Current applications and challenges of circulating tumor DNA (ctDNA) in squamous cell carcinoma of the head and neck (SCCHN). Cancer Treat Rev. 2020;85:101992. doi:10.1016/j.ctrv.2020.101992.
6. Haystack MRDTM: Proven clinical utility. Haystackmrd.com. Accessed May 23, 2024. https://haystackmrd.com/haystack-mrd-proven-clinical-utility.
About the Author

Hillary Sloane, PhD
is the Director of Medical Affairs at Haystack Oncology, a Quest Diagnostics company. In her role, Dr. Sloane provides key clinical strategy support and primary oversight for the clinical development of Haystack’s cutting edge circulating tumor ctDNA minimal residual disease technology. She engages closely with the medical community to establish and execute clinical development initiatives that specifically address current and future needs to improve patient care. Hillary has an extensive background in molecular diagnostics and liquid biopsy, with experience in clinical development and medical scientific affairs roles in the oncology diagnostics industry. She holds a PhD in bioanalytical chemistry from The University of Virginia.