The need for early detection in ovarian cancer
According to the American Cancer Society, it has been estimated that in 2021, in the United States alone, about 21,410 women will receive a new diagnosis of ovarian cancer (OC), and about 13,770 women will die from this disease. OC ranks 5th in cancer deaths among women, with a 1 in 78 chance of women developing this disease during their lifetime and a 1 in 108 chance of dying from the disease.1
While OC can affect women of all ages, it is rare in young women (under the age of 30), as it is most commonly diagnosed in women over the age of 50 (average diagnosis between 50 and 70).
OCs can be classified into three large groups: epithelial (circa 90% of tumors), germ cell (circa 5% of tumors), and specialized stromal cell (comprising the remnants). Interestingly, germ cell tumors are more common in women in their early 20s, while epithelial (EOC) tumors occur primarily in postmenopausal women, and sex cord-stromal tumors are most common in women in their 50s.
OC has been referred to as the silent killer, since most women are asymptomatic, or present with vague symptoms, including abdominal distention, urinary frequency and pain on surrounding organs from the pressure caused by the tumor mass. These symptoms are easily mistaken for other benign conditions and are, therefore, often dismissed by women as being related to aging, menopause or previous pregnancies. Because of this, more than 70% of OCs are diagnosed only once the disease is already widespread and has progressed to later stages (stage III or IV).
There is a direct correlation between OC stage at presentation and survival. In stage I, when OC is limited to the ovaries, currently available surgery and chemotherapy can cure this disease in up to 90% of cases. Similarly, when the cancer has spread to the pelvis (stage II), 5-year survival reaches 70%. When the cancer has spread to other organs, such as the abdominal cavity in Stage III or outside the abdominal cavity and/or into the liver parenchyma in Stage IV, the cure rate drops to 20% or less.
It is, therefore, evident that early detection of OC could significantly reduce mortality rates and impact long-term disease control.
Diagnosis of ovarian cancer
As previously mentioned, the early detection of OC is, indeed, a crucial element for improving the patient’s survival rate. According to the guideline from American College of Obstetricians and Gynecologists (ACOG) in 2016, the individual patient characteristics, physical examination findings, imaging results (including transvaginal ultrasound and Computed tomography (CT) scans), and serum marker measurements should all be used in combination for the evaluation and management of adnexal masses.
The annual or semiannual pelvic examination of women with suspected OC (from ages 25-35 years) allows physicians to feel the size, shape and consistency of the uterus. This approach may be limited due to the physician’s difficulty (or sometimes impossibility) to feel any change in the uterus in early stage ovarian cancers.
Transvaginal ultrasound (TVUS) is the most common imaging test used to evaluate adnexal structures, and it helps in identifying the cancer through morphological changes associated with OC. This approach can help find a mass in the ovary, but it is not capable of differentiating between a benign mass or a cancerous one. CT scans, on the other hand, are not capable of identifying small masses, but only larger tumors.
Given the importance of early diagnosis and the promising effectiveness of biomarkers in OC triage, there has been a great effort to develop novel serum biomarkers and triage serum marker algorithms, with the precise goal of detecting OC at earlier stages, as well as improving the accuracy of referral to specialty care.
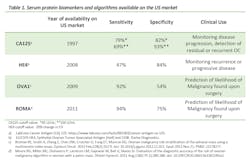
Clinical blood biomarkers currently available in the U.S. market have been shown to improve triage of women with suspected OC and, therefore, reduce mortality rates. The table below (Table 1) shows the serum protein biomarkers and algorithms currently available in the United States.
CA125
Cancer antigen 125 (or MUC16) is the most widely used biomarker for OC detection, and is often considered the “gold standard.”2 First identified in 1981, CA125 was FDA approved in 1997 as a cancer surveillance biomarker for women with a known diagnosis of OC. CA125 is, in fact, clinically used for monitoring chemotherapy responses, for detection of recurrence, and for improving clinical trial design.3
Interestingly, recent studies have also indicated that changes in CA125 levels may occur 18 months before clinical diagnosis, thus supporting the use of CA125 as a biomarker for early detection of OC.4 However, there are limitations to the use of CA125 as an early detection biomarker. Specifically, although high levels of CA125 are present in 80% of advanced stage OCs and strongly correlate with specific subtypes of OC (including serous and endometrioid),5 serum CA125 appears to be elevated in only 50% of patients with Stage I ovarian cancer.6 Furthermore, CA125 is also elevated in common benign gynecological conditions (such as endometriosis, follicular cysts, cystadenomas, ovulatory cycle, and pregnancy), especially in premenopausal women.3 All of these factors significantly affect CA125’s sensitivity and specificity as a useful stand-alone biomarker for the early detection of OC. As a result, CA125 should be used in combination with patient data and imaging.7 CA125 may be more useful in conjunction with one or more other tumor biomarkers. Additional markers could play a role if, when used with CA125, they identify some carcinomas missed by CA125 (i.e., they improve sensitivity).
HE4
In 2009, the U.S. Food and Drug Administration (FDA) approved the Human epididymis secretory protein E4 (HE4/WFDC2) as a biomarker for monitoring both recurrence (HE4 detects EOCs 2 to 3 months earlier than CA1258), as well as disease progression.
As with most biomarkers, HE4 is expressed in both normal and malignant tissues. Specifically, its expression has been observed in the epithelium of fallopian tubes, endometrium and in the endocervical glands, as well as the epithelia of the respiratory tract, renal convoluted tubules and salivary glands.9
High levels of HE4 have been observed in specific subtypes of OC, such as serous (93-100%), endometrioid (80-100%), and clear-cell carcinomas of the ovary (50-83%), while it is absent in mucinous OC.9 This may suggest the role of HE4 in differentiating and better characterizing specific histological OC subtypes.10 Besides OC, HE4 is also elevated in other malignancies, including mesothelioma, lung, endometrial, breast, gastrointestinal, renal and transitional cell carcinomas.11
Interestingly, HE4 is less frequently elevated in benign ovarian tumors (both in pre- and post- menopausal women), serous cysts, teratomas, fibromas and inflammatory lesions, compared to CA125.9
Furthermore, HE4 (as well as ROMA) have shown to be very useful tools to exclude malignancies in endometriosis.12
Several studies have reported that HE4 alone is a better biomarker than CA125 alone in the diagnosis of OC.13 Specifically, the higher sensitivity of HE4, compared to CA125, in premenopausal women14 allows for better diagnosis of early-stage OC and borderline tumors.15 Moreover, the higher specificity also allows the use of HE4 in monitoring OC recurrence.16
Interestingly, HE4 used in conjunction with CA125 yielded significantly greater sensitivity (at a set specificity) than either marker alone, or any other dual combination of markers.15
OVA1
Over the past few years, several algorithms have been developed to help with risk assessment of adnexal masses.
In 2009, OVA1 was approved by the FDA as a new algorithm that combines data from imaging, menopausal status, and a panel of 5 biomarkers: CA125, ApoA1, TTR, Tf and β2-macroglobulin. In 2016, the FDA cleared a second-generation multivariate index assay called OVERA, which combines CA125, HE4, ApoA1, follicle stimulating hormone (FSH) and Tf. Because FSH is part of the panel, there is no need to determine menopausal status. Overall, OVERA presents similar sensitivity and NPV to OVA1 (specifically 91% vs 94% sensitivity respectively and 97% NPV), although performing better for specificity (69% vs 54% respectively) and PPV (40% vs 31%).17
Risk of ovarian malignancy algorithm (ROMA)
In 2011, the FDA approved the ROMA score as a qualitative serum test designed to assess the likelihood of finding malignancies during surgeries in women with an adnexal mass and, thus, allows for better patient triage to centers of excellence.
The ROMA algorithm combines measurements of serum HE4 and CA125 to the menopausal status of the patient (defined by lack of menstruation or clinical signs of menopause for 6 months). The creators of this algorithm revealed a sensitivity of 93.8% (88.9% for pre-menopausal and 94.6% for post-menopausal women) and a specificity of 75% for the diagnosis of EOC.18 Based on their result, the ROMA algorithm successfully classified patients into high and low risk groups with 93.8% of EOC correctly classified as high risk.18
Conclusion
The biggest challenge healthcare providers face when presented with women with adnexal masses, is to correctly assess the risk for OC as early as possible, despite the vagueness (or lack) of symptoms. Currently, there are no recommended screening tests for OC in the United States, due to high false positive and false negative rates, resulting in unnecessary surgeries or delayed treatment.19
In order to overcome this diagnostic challenge, blood biomarkers and algorithms have significantly contributed to overcoming this issue and improving the referral of women with suspected OC to specialized centers.
References
- Key statistics for ovarian cancer. American Cancer Society. Published 2021. https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html. Accessed May 4, 2021.
- Høgdall E. Cancer antigen 125 and prognosis. Curr Opin Obstet Gynecol. 2008;20(1):4-8. doi:10.1097/GCO.0b013e3282f2b124.
- Sarojini S, Tamir A, Lim H, et al. Early detection biomarkers for ovarian cancer. J Oncol. 2012;2012. doi:10.1155/2012/709049.
- McIntosh MW, Drescher C, Karlan B, et al. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol Oncol. 2004;95(1):9-15. doi:10.1016/j.ygyno.2004.07.039.
- Bast RC, Lu Z, Han CY, et al. Biomarkers and strategies for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2020;29(12):2504-2512. doi:10.1158/1055-9965.EPI-20-1057.
- Chandra A, Pius C, Nabeel M, et al. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019;8(16):7018-7031. doi:10.1002/cam4.2560.
- Kumarasamy C, Madhav MR, Sabarimurugan S, et al. Diagnostic and prognostic role of HE4 expression in multiple carcinomas: A protocol for systematic review and meta-Analysis. Med (United States). 2019;98(28):1-6. doi:10.1097/MD.0000000000015336.
- Ueland F. A Perspective on ovarian cancer biomarkers: Past, present and yet-to-come. Diagnostics. 2017;7(1):14. doi:10.3390/diagnostics7010014.
- Nowak M, Janas Ł, Stachowiak G, Stetkiewicz T, Wilczyński JR. Current clinical application of serum biomarkers to detect ovarian cancer. Prz Menopauzalny. 2015;14(4):254-259. doi:10.5114/pm.2015.55887.
- Nguyen L, Cardenas-Goicoechea SJ, Gordon P, et al. Biomarkers for early detection of ovarian cancer. Women’s Heal. 2013;9(2):171-187. doi:10.2217/whe.13.2
- Galgano MT, Hampton GM, Frierson HF. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19(6):847-853. doi:10.1038/modpathol.3800612.
- Zapardiel I, Gorostidi M, Ravaggi A, et al. Utility serum marker HE4 for the differential diagnosis between endometriosis and adnexal malignancy. Int J Gynecol Cancer. 2016;26(1):52-55. doi:10.1097/IGC.0000000000000579.
- Bristow R, Jordan. Ovarian cancer biomarkers as diagnostic triage tests. Curr Biomark Find. Published online 2013:35. doi:10.2147/cbf.s30228.
- Holcomb K, Vucetic Z, Miller MC, Knapp RC. Human epididymis protein 4 offers superior specificity in the differentiation of benign and malignant adnexal masses in premenopausal women. Am J Obstet Gynecol. 2011;205(4):358.e1-358.e6. doi:10.1016/j.ajog.2011.05.017.
- Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108(2):402-408. doi:10.1016/j.ygyno.2007.10.017
- Langmár Z, Németh M, Vleskó G, et al. HE4 – a novel promising serum marker in the diagnosis of ovarian carcinoma. Eur J Gynaecol Oncol. 2011;32(6):605-610.
- Coleman RL, Herzog TJ, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol. 2016;215(1):82.e1-82.e11. doi:10.1016/j.ajog.2016.03.003.
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112(1):40-46. doi:10.1016/j.ygyno.2008.08.031
- Stacy Simon. FDA warns against ovarian cancer screening tests. American Cancer Society. 2016. https://www.cancer.org/latest-news/fda-warns-against-ovarian-cancer-screening-tests.html. Accessed May 4, 2021.
About the Author

Francesca I. De Simone, PhD
is a scientific affairs specialist at Fujirebio Diagnostics.