The 2024 Medical Laboratory Observer (MLO) State of the Industry (SOI) survey on Disease Management focused on diabetes (type 1 and type 2) and sexually transmitted infection (STI) testing. Alongside the quantitative results, we include commentary from BD, Hologic, Nova Biomedical, Revvity and Roche Diagnostics on diabetes and STI sampling and testing trends and innovations.
Among the survey respondents, the majority (63%) hold laboratory manager, administrator or supervisor positions and are employed by health system or hospital labs (63%). When asked how many employees work in their lab, 30% reported 1-10, 25% 21-50, 23% 11-20, 13% more than 100, and 10% between 51-100.
Key survey findings are as follows:
· 46% of respondents report a moderate or significant increase in diabetes testing
· 50% of respondents report a moderate or significant increase in STI testing
· Increased positive results for specific STIs as reported by lab professionals surveyed:
o Bacterial vaginosis: 11% increase (28% in 2024, compared with 17% in 2023)
o Gonorrhea: 6% increase (23% in 2024, compared with 17% in 2023)
o Trichomoniasis: 6% increase (15% in 2024, compared with 9% in 2023)
Diabetes testing trends
When asked if their lab has seen an increase in diabetes testing over the past 12 months, 46% of respondents reported a moderate or significant increase, while 55% said they have not seen an increase.
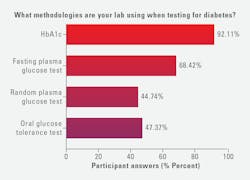
Regarding methodologies for diabetes testing, most lab professionals (92%) reported using HbA1c, followed by fasting plasma glucose test (68%), oral glucose tolerance test (47%), and random plasma glucose test (45%).
Turning to testing methods for HbA1c, nearly half of survey respondents (47%) report using immunoassay as their methodology, 32% enzymatic testing, 18% cation exchange HPLC, 8% capillary separation, and 5% boronate affinity chromatography.
For those labs performing both the fasting blood glucose test and HbA1c test as screening measures for types 1 and 2 diabetes, 40% report using more HbA1c tests, 38% more fasting blood glucose tests, and 23% said they use both testing methodologies equally.
With the Centers for Disease Control and Prevention (CDC) reporting that 38.4 million people in the U.S. have diabetes,1 MLO questioned lab professionals on whether their current diabetes testing procedures are meeting their patient populations’ needs/expectations.
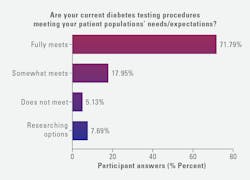
Nearly three-quarters of lab professionals surveyed (72%) said their procedures fully meet their populations’ needs/expectations, 18% said they somewhat meet them, 8% said they are researching options, and 5% said their testing procedures do not meet their patient populations’ needs/expectations.
Among those survey respondents who are researching options, one commented on how they are currently sending out HbA1c testing but have purchased an analyzer to be validated in the near future.
Innovations in diabetes testing
Point of care (POC) testing
According to Dennis Begos, MD, FACS, senior medical director, Medical and Scientific Affairs, Nova Biomedical, while the company is primarily known for its glucose testing, they are now focusing more on chronic disease management, specifically outside of the hospital. He stated:
“With almost 80% U.S. market share in glucose testing inside of the hospital, we are still heavily focused on that, but what we are seeing as a trend is point of care (POC) testing outside of the hospital setting. Laboratory testing is moving away from people having to go to the hospital or outpatient lab to have blood drawn to have everything done when they visit their PCP in the office. This way, with a finger stick, they can have their HbA1c and cholesterol checked, as well as other testing, such as coagulation and kidney function testing.”
As Begos pointed out, this approach is less expensive, more convenient and has been shown to have improved outcomes because the PCP and patient have results immediately and can discuss diagnosis, preventative actions and/or course of treatment during the care visit.
“Certainly, in the next five years we will see more availability of outpatient fingerstick blood testing,” said Begos. “In Italy and Spain, they are doing a lot of this testing in pharmacies. For kidney disease, patients who are on medications that may be harmful to the kidneys or are diabetic and at risk for kidney disease, the pharmacist can test for kidney function and adjust medications as needed.”
Speaking to the role of hospital pharmacies when it comes to POC testing, Begos stated:
“If a POC test is abnormal, it will have to be verified by a central lab. POC testing will increase the number of people tested overall, so I think it will increase labs’ testing volumes overall as well.”
Neonatal detection
The CDC estimates that 304,000 children and adolescents under 20 years of age in the U.S. have diagnosed type 1 diabetes, with incidence significantly increasing among this population during the most recently recorded period (2002-2018).1
Chief Scientific Officer at Revvity Dr. Madhuri Hegde, spoke to the company’s development of an assay for neonatal detection of type 1 diabetes, noting how the “future of helping those with type 1 diabetes will soon come down to a drop of blood.” She stated:
“Here at Revvity, our singular focus is constantly figuring out how we stand side by side with our customers as a scientific partner and help revolutionize life by solving some of the most complex diseases and challenges that we face today. This includes type 1 diabetes. When we talk about the life-saving impact that Revvity’s newborn screening capabilities have on millions of babies around the world – it begins with just one drop of blood from a heel prick.”
Revvity designs and manufactures dried blood spot – or DBS cards – which enable blood samples to be transported to labs and analyzed for life-altering, metabolic diseases. According to Dr. Hegde, the company has screened more than 800 million newborns for rare diseases using DBS, saving the lives of 700,000 on a global scale for nearly four decades.
“It's something we’re very proud of,” she commented. “It's amazing to see how much this technology has meant in blood sample collection and logistics, combining scientific precision, efficiency, and affordability, to provide important diagnostic answers for families. We are enthusiastic about the potential to screen for type 1 diabetes with the use of DBS.”
Dr. Hegde described the benefits of using DBS to test all ages for type 1 diabetes:
“We would have a non-invasive sample collection and unique logistical advantage that also allows for a centralized screening strategy with the possibility of developing different sample collection strategies until reaching the home kit concept. This ultimate goal will give answers to the patient sooner for quicker and more effective health intervention.”
Sexually transmitted infection (STI) testing trends
MLO revisited the topic of STI testing in this year’s survey, having included it in the 2023 State of the Industry (SOI) survey on Disease Management. When asked if their labs had seen an increase STI testing in the past 12 months, 50% of respondents reported a moderate or significant increase, compared with 46% in 2023. The other half of respondents (50%) reported no increase.
With the CDC reporting that one in five Americans have an STI,2 access to testing is critical to diagnosis and preventing the spread of infections.
“Labs play a critical role in delivering accurate results that help healthcare providers choose the appropriate treatment for their patients,” said Jennifer Schneiders, PhD, president, Diagnostic Solutions at Hologic. “The most common STIs identified in the most recent CDC Surveillance Report were chlamydia and gonorrhea. Strategically combining molecular diagnostic testing for similar infections can help accurately identify the cause in a timely manner.”
“It’s never been more important to provide accurate testing information quickly to doctors and their patients for assessing risk and helping to diagnose, monitor and treat patients,” said Denise Heaney, chief medical partner for molecular solutions, Roche Diagnostics. “Access to screening and diagnostic testing is often the first line of defense. Innovative, reliable and accessible testing can transform STIs into manageable conditions.”
“Because many STIs are asymptomatic or have mild symptoms that can be mistaken for other conditions, people are often unaware of their infection status and do not seek testing or care,” said Alyssa Davis, MS, PA-C, medical science liaison II, U.S. Region, Women's Health & Cancer, Scientific Affairs, BD. “As a result, many STIs remain undiagnosed and untreated, posing a threat to individual and public health, so it’s important to make testing easier and speed up the time to diagnosis so that patients with an infection can start the right treatment right away and reduce potential spread.”
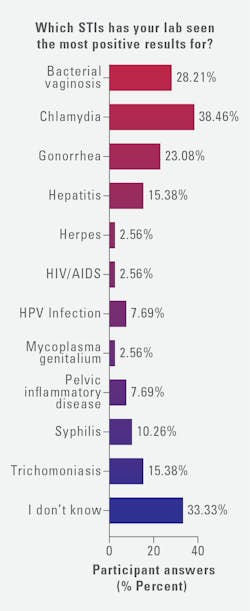
Looking at testing for specific STIs, those surveyed reported the most positive results for chlamydia (38%), bacterial vaginosis (BV) (28%), and gonorrhea (23%). This was followed by hepatitis and Trichomoniasis (both at 15%), syphilis (10%), pelvic inflammatory disease (PID) and human papillomavirus (HPV) infection (both at 8%), Mycoplasma genitalium (3%), and herpes and HIV/AIDS (both at 3%).
Comparing this year’s survey results to last year’s results, the data showed trends in positive results for BV, with an 11% increase (28% in 2024, compared with 17% in 2023), gonorrhea with a 6% increase (23% in 2024, compared with 17% in 2023), and Trichomoniasis with a 6% increase (15% in 2024, compared with 9% in 2023).
Reported increases in positive results for the following STIs were also slightly higher: PID (8% in 2024, up from 4% in 2023), hepatitis (15% in 2024, up from 13% in 2023), Mycoplasma genitalium (3% in 2024, up from 0% in 2023), and HIV/AIDS (3% in 2024, up from 0% in 2023).
Trending downward were positive increases for herpes (3% in 2024, down from 9% in 2023), HPV infection (8% in 2024, down from 13% in 2023), and syphilis (10% in 2024, down from 13% in 2023). Reported increases in positive results for chlamydia remained relatively the same, only decreasing 1% (38% in 2024, down from 39% in 2023).
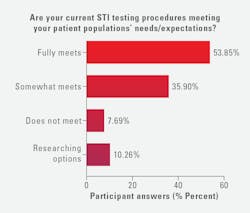
When asked whether their current STI testing procedures are meeting their patient populations’ needs/expectations, more than half of lab professionals (54%) said they fully meet needs/expectations, 36% somewhat meets, 10% said they were researching options, and 8% said their procedures do not meet their populations’ needs/expectations.
Among those researching options for STI testing, one lab professional noted how their lab was planning to switch from a wet prep panel to a molecular vaginosis panel. Another said they had recently validated Cepheid’s multiplex vaginal panel (MVP).
Supply challenges
Turning to supply challenges, 31% of those surveyed say they have had trouble acquiring swabs for STI testing, 23% assays, 10% blood specimen tubes, and 5% urine collection devices.
When comparing these findings to last year’s survey results, more lab professionals are struggling with the acquisition of assays for STI testing (23% in 2024, up from 18% in 2023), while fewer face challenges acquiring blood specimen tubes (10% in 2024, down from 36% in 2023), swabs (31% in 2024, down from 55% in 2023), and urine collection devices (5% in 2024, down from 27% in 2023).
Innovations in STI sample collection and testing
Sample collection modalities
Davis said one way to make STI testing easier is through self-collection of samples, where a person in a healthcare setting - not necessarily a doctor’s office, but a clinic, lab or even retail pharmacy - performs a simple swab themselves, in privacy, which is then tested for multiple STIs using just the one sample.
“Improving ease and access by expanding the availability of STI testing services, especially in rural and remote areas and among underserved populations, can go a long way,” Davis added. “U.S. Food and Drug Administration (FDA) approved combination tests that detect the most prevalent non-viral STIs - Chlamydia trachomatis, Neisseria gonorrhoeae and Trichomonas vaginalis - simultaneously and separately allow clinicians to get results for all three from one sample, and that’s easier for the patient, too.”
“Our collection device helps streamline testing for providers and labs by testing up to seven infections and disease states with one vaginal sample,” said Schneiders. “We are able to not only test for chlamydia and gonorrhea but vaginitis and other STIs such as Mycoplasma genitalium. This allows healthcare providers to identify the cause with one sample and offer the right treatment as quickly as possible.”
Heaney pointed to the FDA’s approval of the Roche HPV self-collection solution as a recent example of how innovation in STIs can potentially change the testing landscape.*
“This is an important innovation in cervical cancer diagnostics that expands access to HPV screening options by enabling women and individuals with a cervix to privately collect their own vaginal sample in a healthcare setting,” said Heaney. “The sample is then placed by trained personnel into a preservative solution and transported in controlled conditions to the laboratory where it is analyzed with the Roche cobas HPV test, which runs on the cobas 4800 and the fully automated cobas 5800/6800/8800 Systems.”
“This solution allows for broader access to care and could support the lessening of health disparities in cervical cancer, which is more prevalent among Black women and Hispanic-Latina women,” Heaney added. “Black women are less likely to be diagnosed with cervical cancer, yet they have the highest death rates compared to all other racial and ethnic groups due to profound survival disparities, which may be attributed to a lack of access to timely, high-quality care.”3
*HPV self-collection is FDA-approved but not yet available for sale in the U.S.
Testing modalities
“Once the sample has been collected, the speed and accuracy of diagnosis is an important factor, so the ability to run the tests through diagnostic devices quickly makes a difference,” said Davis. “Samples are often sent to labs for processing through high-throughput molecular diagnostics platforms which can deliver results in about a day.”
Schneiders, said she is seeing many healthcare providers (HCPs) moving to nucleic acid amplification testing (NAAT) for detecting BV, Candida vaginitis (CV), and Trichomoniasis (TV).
“Compared to conventional testing methods, NAATs offer objective, comprehensive and accurate results with the first test,” said Schneiders. “NAATs are FDA cleared and recognized by the CDC and the American College of Obstetricians and Gynecologists (ACOG). Testing all three infections together is important because they are all are similar, yet each cause has its own characteristics, consequences and treatment recommendations. Identifying the specific cause of a patient’s vaginitis, including those with mixed infections, can allow women to be more effectively diagnosed and then treated.”
According to Heaney, labs need a broad-based portfolio for early diagnosis to treatment monitoring, especially in areas for syphilis, Chlamydia trachomatis, Neisseria gonorrhoeae and HPV. She emphasized the need to provide fast-turnaround results in small to large laboratories and near-patient settings, such as emergency departments and urgent care clinics.
“Roche aims to improve access to diagnostics by being where the laboratorian, clinician and their patients most need testing, said Heaney. She noted how this could include “home testing, point-of-care testing, and multiplex testing for multiple STIs.”
“This is especially important as we know that the emergency department visit or urgent care clinic visit may be the only clinician visit that patient has for a year, or two, or more,” Heaney continued. Clinicians need to diagnose and potentially start treatment before that visit has concluded.”
Davis spoke to additional challenges faced by laboratory teams today and the importance of testing modalities that support efficiency. She stated:
“We all know the pandemic exacerbated the challenges labs face with regards to staffing, volume and demand. Reducing manual processes and increasing ‘walk-away time’ by eliminating the need to sort specimens frees up laboratorians’ time for other critical lab processes.”
She commented on the importance of modalities that speed time to diagnosis, as well as those that enable labs to test multiple types of specimens for different types of infections simultaneously.
“COVID-19 taught us all how important point of care testing is, so the ability to provide same-visit lab-quality results within 15-20 minutes using a table-top device is very valuable,” said Davis. “Combine self-collection with same-visit speed of diagnosis using a single test that detects multiple kinds of infections — that’s where STI testing needs to go.”
References
1. CDC. National Diabetes Statistics Report. Diabetes. Published June 6, 2024. Accessed July 2, 2024. https://www.cdc.gov/diabetes/php/data-research/index.html.
2. CDC. STD news media resources. NCHHSTP Newsroom. Published May 20, 2024. Accessed July 2, 2024. https://www.cdc.gov/nchhstp-newsroom/resources/std.html.
3. Cohen CM, Wentzensen N, Castle PE, et al. Racial and Ethnic Disparities in Cervical Cancer Incidence, Survival, and Mortality by Histologic Subtype. J Clin Oncol. 2023;10;41(5):1059-1068. doi:10.1200/JCO.22.01424.
About the Author

Kara Nadeau
has 20+ years of experience as a healthcare/medical/technology writer, having served medical device and pharmaceutical manufacturers, healthcare facilities, software and service providers, non-profit organizations and industry associations.