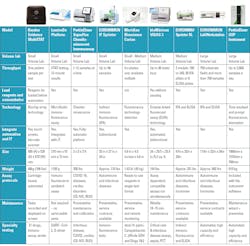
Immunoassay analyzers vary in the size, throughput and technology. As labs are currently scaling the number of analyzers to align with their throughput needs, some of the leading immunoassay analyzers are compared here.
Rosalyn Yalow, PhD, and Solomon A. Berson, MD, won the Nobel Prize in 1977 for their discovery of Radioimmuneassay (RIA), and the technology grew with Cesar Milstein, PhD, and George J. F. Kohler, PhD, winning the Nobel Prize in 1984 for the production of monoclonal antibodies. Since then, a plethora of immunoassays have launched.
The first Curian HpSA assay was for active infection H. pylori testing, which is being followed by additional assays for Campylobacter, C. difficile GDH/Toxin, and Shiga Toxin 1&2.
Randox’s Evidence MultiSTAT has a Stroke Biochip for rapid stroke diagnosis in about 12 minutes, and the LumiraDx Platform is a rapid microfluidic test designed on similar principles as lab analyzer systems in a portable, affordable solution.
For high volume labs, EUROIMMUN’s LabWorkstation enables fully automated and standardized processing, while the PerkinElmer GSP Instrument features a refrigerated compartment for up to 13 cassettes, buffers, tracers, and antibodies.
Considerations when purchasing
Arvind Kothandaraman, General Manager of Specialty Diagnostics at PerkinElmer, suggested labs look beyond current needs, “particularly in a post-pandemic world. Lab leaders will benefit from choosing instruments that require minimal human intervention and training for processing samples, while allowing the option to scale up as needed to meet future demands. Automated immunoassay analyzers enable lab staff to save time, standardize processes and reduce errors. Lab leaders should also consider the support and service options available from various manufacturers, as the one they choose will play an important role in facilitating uninterrupted processing of critical assays.”
The LumiraDX platform and the Curian have no required maintenance. Others models display needed tasks on their screen, and some manufacturers offer maintenance contracts.
Christopher Liddle, Marketing Executive of Randox, advises, “When purchasing an immunoassay analyzer, lab professionals should consider the following: (1.) Automation – an analyzer which offers greater automation reduces the workload of professionals, while reducing the possibility of human error. (2.) Return on Investment – if the analyzer in question can allow the laboratory to function at a faster rate or with greater efficiency, then the laboratory will be able to improve productivity and in turn, increase profitability. (3.) Improved Precision – when purchasing a modern analyzer with the latest technology in comparison to an older product, the newer software will likely allow for more precise results, which will improve the validity of output data within the laboratory.”
For example, Sarah Berger, Senior Manager, Marketing Communications for Diagnostics at Meridian Bioscence, said Curian’s platform can bring “testing closer to the patient for improved clinical efficiencies. The fluorescent technology on Curian provides increased sensitivity and eliminates subjectivity associated with other testing methods.”
Experiences with immunoasay analyzers
Testing for PCT, or procalcitonin, a biomarker that distinguishes bacterial infections from other inflammatory reactions, effectively helps monitor sepsis. John Boreyko, PharmD BCIDP, Infectious Disease Pharmacist at Duke Regional Hospital, said this testing was incorporated, “to aid us in the reduction of both initiating inappropriate antibiotics and deescalating therapy in culture-negative septic patients. bioMérieux was with us the entire time after our business plan was accepted and was instrumental in helping educate our chemistry lab on using the equipment, validating the equipment and helping us to meet CAP guidelines.”
Daniel Feinstein, MD, Medical Director Critical Care at Novant Health, explained, “VIDAS procalcitonin [is] an integral part in antibiotic stewardship and allow[s] physicians to de-escalate and even discontinue or shorten the duration of antibiotics with a lot more confidence, in an algorithmic fashion.”
During Spring 2020, Andre B. Gvozden, MD, a Pediatric Specialist, was introduced to the SARSCoV-2 Antigen Test on the LumiraDx Platform as part of a clinical study. Over the last 10 years, Gvozden has evaluated lots of different point of care instruments/platforms for respiratory pathogens, urine and serum testing. During the use of the platform at his practice, Gvozden reported that he, “experienced zero glitches, which has not been the case with previously assessed point of care tests. With other tests, it can be frustrating to spend $20-30 on a test, only to end up with an invalid result or error.”
Avoiding that frustration, patients responded positively to the faster testing, trusting “a result which comes from their doctor,” explained Gvozden. “This gives a sense of confidence.”
Considerations when upgrading
Liddle gives three examples of when a lab may consider upgrading their immunoassay analyzer, listing a financial reason first. “(1.) Alternative Products Offer a Better Return on Investment – If a new product is on the market which offers features that would enhance the laboratory’s testing capabilities, then it would be beneficial for the lab to invest in an upgrade in order to further improve its productivity.
“(2.) Increased Wear & Tear – If the analyzer has been used over a long period and is now suffering from increased wear and tear, resulting in growing downtime which negatively impacts performance, then a laboratory should consider an upgrade to prevent this downtime and constant maintenance requirements from impacting their performance. (3.) Outdated Software – Many older analyzers will not have the software available in more modern analyzers. Outdated software may increase processing time and act only to negatively impact throughput time. Therefore, the laboratory may want to upgrade to an analyzer which offers modern technology to increase throughput time and overall efficiency.”
According to Brian Duchateau, PhD, Vice President of Scientific Affairs, LumiraDx, Waltham, MA, USA, “Rather than upgrading an immunoassay analyzer, labs should consider where it makes sense to decentralize testing with next generation POC analyzers that combine speed, ease-of-use, and lab-comparable performance.”
Greg Stock, Vice President of Commercial Operations, EUROIMMUN US, advised, “Laboratory professionals should always keep in mind what the future might bring to their laboratory. Purchasing for today’s needs may not position the laboratory appropriately for future growth and expansion.”