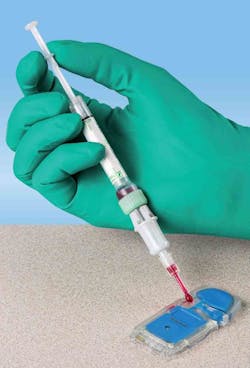
POC collection kit
Sarstedt’s S-Monovette point-of-care (POC) Collection Kit provides controlled venous sampling from vascular access lines and precise dispensing for POC testing. This dual technology reduces POC test rejection by helping prevent overfilling, underfilling, or air bubbles in the cartridge. This results in decreased turnaround times and improved patient outcomes. After POC dispensing, the analyzer-ready S-Monovette tube can be sent directly to the lab if confirmation testing is required, facilitating patient blood management. Sarstedt, Inc.
POC hemoglobin analyzer
The Diaspect handheld, reagent-free hemoglobin analyzer is cleared for use in point-of-care (POC) and Certificate of Waiver settings such as physicians’ offices, clinics, and other non-traditional laboratory locations. The DiaSpect provides users with accurate hemoglobin measurements (precision: CV ≤1%) within two seconds of its whole blood-filled cuvette being inserted for analysis. This ensures immediate and robust hemoglobin results for patient health checks and anemia screening at the POC. Based on its FDA categorization, the lightweight and palm-sized analyzer can be used by a wide range of healthcare personnel. It is highly user-friendly, requiring minimal training. The user collects a capillary or venous blood sample of 10 µL in the cuvette before inserting straight into the analyzer. DiaSpect is factory calibrated against the HiCN reference method in accordance with ICSH and is ready to use with no re-calibration or maintenance necessary. Its reagent-free, disposable cuvettes have up to 2.5 years shelf life, are unaffected by temperature or humidity, and can be stored from 0°C to 50°C. Short-term storage is possible at 30°C to 70°C for 24 hours. These features makes the device highly suited for hemoglobin testing in a range of locations, environments, and climatic conditions. EKF Diagnostics
HIV and HCV rapid antibody tests
The OraQuick platform can save time for staff and patients. OraQuick ADVANCE Rapid HIV-1/2 Antibody Test is FDA-approved for use with oral fluid, fingerstick, or venous whole blood and plasma. OraQuick HCV Rapid Antibody Test is an FDA-approved, CLIA-waived, and CE-marked point of care test for HCV. Both of the OraQuick devices use the same protocol, design, and procedure to test for HIV and HCV, all from one fingerstick with results in 20 minutes. OraSure
Blood analysis system
Molecular option for infectious disease testing
Silaris is a molecular diagnostic test that offers the simplicity, convenience, and procedural familiarity of traditional visual read rapid tests, while providing the superior sensitivity and specificity of true laboratory‐based PCR testing for point‐of‐care (POC) infectious disease diagnosis. The system utilizes proprietary OscAR technology within a microfluidic cassette, allowing facilities to implement an affordable, high performing, CLIA-waived, molecular testing option to diagnose and treat patients quickly and effectively. The platform is cleared for the detection of the influenza A and B virus, showing a sensitivity of 97 percent for flu A and 94 percent for flu B. Sekisui
Urinalysis dipstick control
Quantimetrix has announced the launch of the Dipper POCT Single-Use Liquid Urinalysis Dipstick Control. Dipper POCT has a temperature stability of three months and three years refrigerated from date of manufacture. It is made with a simulated human urine matrix, and its pouch design allows users to visually verify full immersion of the dipstick. That minimizes the risk of contamination and reduces the volume required for testing. Dipper POCT is designed for use in every testing environment, including central labs, reference labs, nursing stations, and doctors’ offices. Its thoughtfully designed packaging makes it easy to distinguish between levels and know when it’s time to reorder. Quantimetrix