Digital pathology and clinical testing – Considerations for a successful integration
Digital pathology has very quickly simply become pathology. It is not considered the future and there is no longer a question of whether it will take root, it is being deployed and used in laboratories across the globe. Those who have not already deployed some form of digital technology are either in implementation, assessing the available solutions, or developing their plans for beginning their digital path.
There are several things to consider when implementing a digital solution and workflow and not the least of these are the integration into a clinical testing environment. In this article, I will be focusing on the key items to examine and provide a framework for consideration. This framework will vary depending upon the type of laboratory/facility and the use cases (just clinical; clinical and education; clinical and research; clinical, education, and research; etc.) but it can be adapted. To realize the true benefits of digital, flexibility is key - both in the vendor and solution you select — and in your implementation and use of it. Keep in mind that the focus in this article is for clinical integration.
Items to evaluate internally
Workflow: What is the current, manual workflow. Gathering and documenting the current process from the time a requisition and the associated specimen(s) are received until the case is signed out is key to outlining which areas need to be adapted for digital workflows. The goal can either be to closely replicate the existing workflow or to optimize workflows with digital. These are two very different approaches, and each should be considered when determining your business goals and objectives. The decision here will also have an impact on the solution(s) you select and the way they are implemented.
There are many small items in this category that have a major impact on integration — such as slide labels, naming conventions, barcodes, case distribution, how to work in both glass and digital workflows simultaneously (the assumption is that you will not be going to a 100% digital workflow immediately).
Integration of AI algorithms: Will you be using artificial intelligence (AI) algorithms? Are they approved by the U.S. Food and Drug Administration (FDA) or will they be validated as an aide to the pathologist only? You will need to identify which cases, customers, or pathologists they will be used with. What is your business model for these? Will you be charging extra for their use if you are doing the professional component (PC) work as outreach business or as consultation, for example?
Phased deployment: You will need to determine if you are doing a phased approach to your deployment/integration (highly recommended). With a phased approach you can decide how you would like that to be broken out and some of the more common choices are: By case type, by specimen type, by customer, by pathologist(s), by percentage of overall cases (i.e., 20% of all case volumes to start then increasing over a set time frame).
Some organizations will decide that they would like to break the workflow into a phased approach, depending upon the completeness and robustness of the solution they select. If a full digital solution, then you may decide to start with just integrating the workflow portion for case accessioning and continue to do the final reporting in your laboratory information system (LIS); then, for a Phase II, do the full bidirectional integration that includes results.
Location: Starting with the location(s) of your scanners to the locations you are supporting, there are physical and virtual integration considerations. This includes the types of work you are performing — is it global, technical only, professional only, internal only, or do you have outreach clients and/or do you do consults? In these cases, there are other ways to tackle the integration within your organization’s clinical work. For example, if you are doing the technical component (TC) work, then providing secure access to your customer’s cases for them to perform the PC side digitally can have a significant impact on cost savings and turnaround times. The same is true if you are doing the PC side of the work and someone else is performing the TC side. By simply having an interfaced scanner at their location, the cases will be available to you for interpretation as soon as they make and scan the glass. Again, avoiding the packing, shipping/courier, and tracking.
There are also the lab applications involved to consider if you are a multi-site entity. Do you have multiple electronic medical records (EMRs), LISs, etc.? Do they all need to be integrated, or do you already have some consolidated in your ordering and reporting processes implemented? Be sure that the digital solution (and vendor) you select are flexible enough to accommodate different scenarios so there is minimal or no reworking required. This will equal less disruption and faster deployments.
Validation: Do not forget your validation as part of the clinical-use process. Some solutions can help automate this as part of their capabilities. However, you will need to have your cases set for the validation process, and this can be done simultaneous to your implementation, saving a great deal of time.
Items to evaluate externally
Integration of AI algorithms: Which algorithms are you looking to incorporate? From which vendor(s)? There are several vendors who provide machine learning (ML), image analysis (IA), and AI algorithms for a variety of uses from diagnostic aids to tumor identification, counting, and metastasis prediction to quantification and grading. They come in first read (precalculated), ROI (region of interest invoked) and second read (QA/QC). When identifying which one(s) you are interested in incorporating within your organization, things to consider are the use cases and their ability to be seamlessly integrated within your workflow platform. It does not provide a large advantage to have to use each different algorithm in a separate application, window, or workflow.
Are you looking to develop your own? There are certainly scenarios where you may have the desire or a unique assay in house that you would like to leverage AI for and there are also vendors who provide a platform that enables you to easily create and validate your own for internal use.
Lab application integration: Not all applications, especially older or legacy applications, are as open and readily able to integrate well with other applications. This can be a bit of a limiting factor in the useability and true interoperability between your digital solution and your LIS. However, there are definitely ways to work around this. Many entities are working with their LIS and EMR vendors to encourage better interoperability and others are actually making changes to more current, open technologies. There are large gains in efficiency and uses of digital and AI even with truncated interfacing options from less open LISs. However, the significant benefits and gains are in a truly interoperable, bidirectional ecosystem.
Image storage and archival: As of now, the requirement is still for storage of glass, not digital images. And though many of us believe it is only a matter of time before that changes, the decisions regarding whether to store, what to store, and how long to store are those of the laboratory or administration. I have seen clients who choose not to store beyond the completion of the case — they feel that if they need to rescan, they will just recall the glass and rescan. I have seen clients who take advantage of the digital solution provider’s long term (archival storage) for anywhere from 10 years to indefinitely and then there are those who prefer to offload long-term storage to a third party such as Microsoft Azure or Google. There is no wrong answer. There is only the planning and understanding of the costs and benefits of each form. If utilizing a third-party information management system vendor, you will want to ensure that your vendor is able to integrate bidirectionally (sending and recalling) those images for your historical cases.
Conclusion
Remember, the adoption and use of digital workflows and AI in clinical diagnostics and laboratories is at its inception point. Choosing a vendor and solution that is flexible, nimble, easily customizable and robust are key for your ability to continue to grow on that platform as the technological advances will continue to evolve and be developed for decades to come.
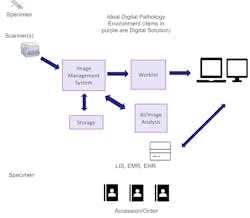
Below is an image of an ideal, full digital framework and environment.About the Author

Lisa-Jean Clifford
Lisa-Jean Clifford has been a noteworthy leader in the high-tech healthcare solutions space for more than two decades. Lisa-Jean’s passion for making a positive impact on the lives of patients through technology can be traced back to her tenure at McKesson and IDX, now GE Healthcare, where she served in vital business development and marketing roles, and to Psyche Systems, an LIS solution provider, where she was the CEO for eleven years. She is currently the President at Gestalt Diagnostics.
Now, recognized as an industry expert, she actively participates in numerous boards including the Association of Pathology Informatics where she serves as President and MLO’s Editorial Advisory Board. She is widely published in many top laboratory publications and noteworthy news sources, such as Forbes, CAPToday, Medical Laboratory Observer, and Health Data Management. Also, she is a highly sought-after speaker and focuses on delivering valuable content in critical areas such as lab automation including software and interoperability, digital pathology, AI in pathology, lab informatics, oncology, and women’s health.