U.S. regulatory clearance for clinical flow cytometry is a breakthrough for leukemia and lymphoma patients
By the time the FDA convened a Public Workshop on Clinical Flow Cytometry in Hematologic Malignancies1 in February 2013, flow cytometry was already a well-established means of evaluating patients with known or suspected leukemia or lymphoma. Despite the lack of any FDA-cleared assays and the consequent requirement for laboratory-developed tests (LDTs), the rapid turnaround times and highly detailed and reproducible results produced by flow cytometry had driven widespread adoption by both clinicians and laboratorians,2 beginning in the late 1980s. So why was the FDA holding a public workshop? What issues did the agency and other stakeholders find to be so urgent as to merit this effort?
Powerful but problematic
Flow cytometric immunophenotyping was first developed by immunologists in the late 1960s and early 1970s as a research tool that enabled their study of the immune system. Had the AIDS epidemic not required the urgent adoption of this technology by clinical laboratories in order to monitor patients’ CD4-positive T helper cell counts in the mid-1980s, flow cytometry might never have been widely adopted for the assessment of leukemias and lymphomas. Once the technique was available to laboratorians, however, its potential utility was apparent. Initially working with reagents labeled for research use only (RUO), immunologists and hematopathologists in laboratories all over the world independently developed in-house assays and expertise that they shared via professional organizations. Vendors that had previously supplied the research market rapidly moved to provide easier-to-use reagents, cytometers capable of analyzing more parameters simultaneously, and more intuitive analysis software suitable for the clinical market.
In 1997, the FDA created a new regulatory category: Analyte Specific Reagents (ASRs).3 Creation of the category was driven by the marked increase in clinical demand for high- complexity LDTs, particularly flow cytometric immunophenotyping and molecular diagnostics: RUO-labeled reagents were of uncertain quality and vendors could not bring IVDs to market quickly enough to meet the demand.
ASR labeling assured laboratories of consistent reagent quality and addressed concerns of patients and insurance companies over the use of RUO-labeled reagents, but also resulted in some unintended consequences as the result of two specific requirements of the labeling: vendors could not market ASR combinations, nor could they provide information that might assist laboratories in combining ASRs. Given that flow cytometric immunophenotyping’s power relies specifically on the simultaneous multiparameter assessment of thousands of individual cells, these requirements presented a challenge to both laboratories struggling to design their own individual LDTs and to vendors possessed of abundant expertise but prevented from sharing it.
The FDA’s position was understandable: high-complexity LDTs could only be performed by laboratories with sufficient expertise to develop and validate their own assays. If vendors assumed any of this burden, then the laboratory did not necessarily have the required expertise.
Guidelines and consensus
Recognizing the need for guidance in the absence of any FDA-cleared in vitro diagnostic (IVD) assays, expert panels published various guidelines and expert recommendations. The 2006 Bethesda International Consensus Recommendations on the Flow Cytometric Immunophenotypic Analysis of Hematolymphoid Neoplasia established a core set of antibody specificities but failed to reach consensus as to panel design—that is, which antibodies to combine together in which tubes; the many independently-developed LDTs could not be easily reconciled into a single assay.4 Bethesda also established the clinical indications that warranted flow cytometric immunophenotyping for suspected hematologic malignancies.5
The 2013 ICSH/ICCS Practice Guidelines for Cell-based Fluorescence Assays reinforced Bethesda recommendations and described the fundamental differences in assay performance criteria between quasi-quantitative assays such as lymphocyte subset and stem cell enumeration assays (for which FDA-cleared IVDs existed) and qualitative assays such as leukemia/lymphoma.6 These differences meant that the former could not serve as predicates for the latter.
2013 FDA Public Workshop
The stakeholders who participated in the Public Workshop on Clinical Flow Cytometry in Hematologic Malignancies included the FDA itself, laboratorians, and vendors. The issues highlighted included the increasing complexity of the LDTs in use by that time, many of which used eight- and 10-color flow cytometry (as opposed to the three- and four-color platforms in use when the FDA first established ASR labeling), as well as the inherent drawbacks of LDTs: labor-intense and error-prone manual workflows, lack of standardization, and wasteful duplication of effort among laboratories. The FDA subsequently summarized many of these concerns in its October 2014 Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories: Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs).7
An FDA-cleared IVD for leukemia/lymphoma
As a result of the 2013 workshop, the FDA engaged directly with flow cytometry vendors to discuss the possibility of developing an IVD test. On June 29, 2017, the FDA announced8 approval via the de novo premarket review pathway of the first agency-authorized test for use with flow cytometry to aid in the detection of several leukemias and lymphomas. Alberto Gutierrez, PhD, Director of the Office of In Vitro Diagnostics and Radiological Health in the FDA’s Center for Devices and Radiological Health, described this test as “…a major step forward for the hematology-oncology community. Laboratories and healthcare professionals now have access to an FDA-validated test that provides consistent results to aid in the diagnoses of these serious cancers.”
Education within product labeling
Flow cytometric immunophenotyping for leukemia and lymphoma relies on pattern recognition by trained professionals. The FDA asked the vendor to include in its product labeling a tool that would familiarize users with the expected staining patterns generated by these particular combinations of reagents. The resulting casebook includes 16 illustrative case studies with characteristic findings typical of various lymphoid and myeloid neoplasms as well as cases from patients with clinical and/or laboratory findings that suggest an underlying neoplastic process, but where no immunophenotypic abnormality is identified. Specimen types include peripheral blood, bone marrow, and lymph nodes. Casebook representative cases were selected from clinical trial data and were reviewed, annotated, and interpreted by the author.
Each case includes a clinical vignette that describes the patient demographics and clinical history, case-specific listmode data files for reanalysis by the user of the casebook, specific analysis protocols to be used with the listmode data, and a report showing the analysis with provided protocols. See Figures 1 through 4 for examples of annotated dot plots (of which there are 60 for each case). Each case concludes with a summary that highlights the immunophenotypic findings as well as potential pitfalls. By independently analyzing the downloadable listmode files, users can further reinforce their pattern recognition skills.
Enhancing patient care
The development of the first preformulated IVD antibody cocktails for use in the clinical lab is a direct result of concerns highlighted by the FDA’s 2013 Public Workshop; and their official statements have confirmed its significance for both the hematology-oncology community and patients.
Estimates from the U.S. Leukemia and Lymphoma Society show that approximately every three minutes one person in the U.S. is diagnosed with a blood cancer, and almost 143,000 people are expected to be diagnosed with leukemia and lymphoma alone in 2017. The availability of this new in vitro leukemia and lymphoma (non-Hodgkin’s only) test is a major step forward for those suffering from these serious cancers.
SIDEBAR
Clinical vignette: chronic lymphocytic leukemia/small lymphocytic lymphoma
A 64-year-old male presents with lymphocytosis. A peripheral blood sample is submitted for flow cytometric immunophenotyping using 5 reagent tubes: CD2-FITC/CD56-PE/CD7 ECD/CD5-PC5.5/CD45-PC7.
The results were as follows: Flow cytometric immunophenotyping identifies a phenotypically distinct population of cells with low light scatter properties that express CD19, low density CD20, CD5, and CD45 and display Kappa immunoglobulin light chain restriction. CD38 expression is absent.
Taken together, the findings in this case are most consistent with chronic lymphocytic leukemia/small lymphocytic lymphoma. Note that correlation with clinical and laboratory data is recommended, and that additional immunophenotyping may be warranted.
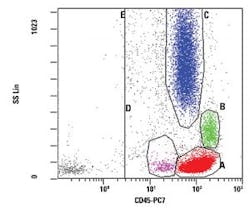
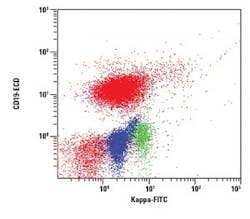
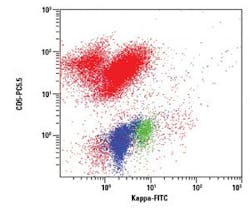
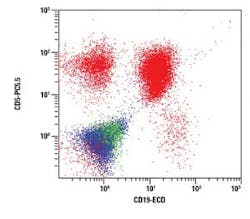
The figures show examples of annotated dot plots
In Figure 1, the dot plot shows the characteristic distribution of the lymphocytes in red, the monocytes in green and the granulocytes in blue. This CD45 vs. Side Scatter dot plot is ungated and shows all events collected. Gate E includes all CD45 positive events and may be used to set a stop count gate during acquisition in order to ensure that sufficient non-debris events are collected. While Gate E may also be used to exclude CD45 negative debris from the analysis, these events should not be ignored when analyzing a case, as some aberrant populations are CD45 negative.
This dot plot permits distinction of the usual populations found in peripheral blood, bone marrow, and lymph node samples, including lymphocytes (Gate A, red), monocytes (Gate B, green), and granulocytes (Gate C, blue). Gate D (pink) is shown here in the area typically occupied by myeloblasts, but may be used to highlight other populations. By applying different colors to the events comprised by each gate, the various populations may be followed throughout the analysis. Gates should be adjusted by the analyst to conform to the naturally occurring separations among the populations, but where no separation is observed an estimate based on experience should be used.
In Figure 2, a Kappa vs. CD19 dot plot is gated on E and shows all CD45 positive events. The prominent CD19 positive population expresses low density Kappa immunoglobulin light chains. A small polyclonal population displaying slightly higher density CD19 is also present. Both populations are red.
In Figure 3, a Kappa vs. CD5 dot plot is gated on E and shows all CD45 positive events. A distinct Kappa/CD5 dual positive population is noted. The CD5 positive/Kappa negative population represents T lymphocytes.
In Figure 4, a CD19 vs. CD5 dot plot is gated on E and shows all CD45 positive events. The CD5 positive/CD19 negative population represents T lymphocytes. The CD19 positive/CD5 negative population represents normal B lymphocytes. The aberrant CD19/ CD5 dual positive population is consistent with the remainder of the analysis.
REFERENCES
- Public Workshop on Clinical Flow Cytometry in Hematologic Malignancies https://wayback.archive-it.org/7993/20161023012242/http://www.fda.gov/MedicalDevices/NewsEvents/WorkshopsConferences/ucm334772.htm.
- Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008.
62 Federal Register: 62243, 26249 (November 21, 1997). - Wood BL, Arroz M, Barnett D, et al. 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry. Part B 2007;72 Suppl 1:S14-22.
- Davis BH, Holden JT, Bene MC, et al. 2006 Bethesda International Consensus recommendations on the flow cytometric immunophenotypic analysis of hematolymphoid neoplasia: medical indications. Cytometry. Part B 2007;72 Suppl 1:S5-13.
- Wood B, Jevremovic D, Bene MC, et al.; on behalf of ICSH/ICCS working group. Validation of Cell-based Fluorescence Assays: Practice Guidelines from the ICSH and ICCS – Part V – Assay performance criteria. Cytometry Part B 2013; 84B: 315–323.
- 79 Federal Register 59776 (October 3, 2014).
- FDA News Release: FDA allows marketing of test to aid in the detection of certain leukemias and lymphomas. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm565321.htm.
Jeannine Holden, MD, MBA, serves as Director of Scientific Affairs & Flow Cytometry Applications Support for Beckman Coulter Life Sciences.