A closer look at the recent FDA safety communication about biotin interference
On November 28, 2017, the U.S. Food and Drug Administration (FDA) issued a safety communication titled, “The FDA Warns that Biotin May Interfere with Laboratory Tests,” which stated:
“The FDA is alerting the public, health care providers and laboratory personnel that biotin can interfere with certain lab tests and cause incorrect lab test results. Some supplements, particularly those labeled for hair, skin, and nail benefits, may have high doses of biotin, which may not be clear from the label. Biotin in patient samples can cause falsely high or falsely low results, depending on the test. Clinical decisions made based on incorrect laboratory test results may lead to patient harm due to inappropriate patient treatment and diagnosis.”
Biotin has always been a part of multivitamins at very low levels (at 30 microgram dose). However, in the past several years, people have been taking biotin at higher levels as over-the-counter supplements (up to 20 mg dose) and prescription dosages (up to 300 mg dose) due to the multifaceted health benefits it offers. With biotin usage increasing and now an FDA safety communication warning about the potential for incorrect lab results, it is critical for the public, healthcare providers and laboratory personnel to understand more about the issue and know the steps that they need to take.
Biotin recommended for various health conditions
Biotin, also known as vitamin B7, is part of the vitamin B complex group, which are nutrients that support the digestive, cardiovascular, and nervous systems. Biotin acts as a co-enzyme in the metabolism of fatty acids, amino acids, and glucose. It has been recommended for the overall health of hair, skin, and nails as well as fetal development. Doctors are also prescribing biotin for several health conditions such as multiple sclerosis, diabetes, hyperlipidemia, dermatitis, depression, Parkinsonism, metabolic acidosis, mitochondrial disease, peripheral neuropathy, and muscle cramps in dialysis patients. In addition to supplements and prescriptions, biotin is also being administered as vitamin therapy via injections or intravenous (IV) drips by itself or as part of other vitamin cocktails.
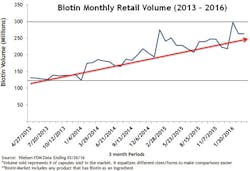
Biotin dosage and consumption have increased. One in three people in the United States takes supplements.1 Biotin supplements, particularly at larger doses, have become increasingly popular. It is estimated that as many as 20 percent of people consume biotin-containing supplements.2 Retail sales of biotin supplements grew more than 260 percent between 2013 and 2016.3 Figure 1 shows only the retail sales volume of biotin. The total sales are probably much higher since these sales figures do not include sales from wholesale warehouses, vitamin and beauty product retail stores, or any online sales from sites where biotin is a top-selling supplement at doses as high as 5 to 10 mg per day.
Why some lab tests are vulnerable to biotin interference
Some lab test developers have designed tests that use the interaction between biotin and streptavidin to capture the analytes in the patient sample; this is usually referred to as the biotin-streptavidin format. This method employs biotinylated antigen or antibody to capture the target analyte in the patient sample. Then, streptavidin-coated microparticles are used to bind these biotinylated components attached to the target analyte, yielding a result based upon the amount of analyte captured. If patients are taking biotin supplements, the free biotin in the blood sample might interfere with this “free capture method” and lead to incorrect lab results and misdiagnosis of patients.
Biotin interference can produce either a falsely lowered or a falsely elevated test result depending on the assay format. In the case of a sandwich assay, where the analyte is “sandwiched” between two antibodies, the results may be falsely lowered due to biotin interference. On the other hand, in the case of a competitive assay format, where the analyte “competes” with the labeled analyte for binding, the results may be falsely elevated due to biotin interference.
Impact of biotin interference on patient results
Although patients taking biotin at levels ranging from 5 mg to 600 mg per day have not experienced adverse effects,4 lab tests that use the biotin-streptavidin “free capture” technique have been documented to produce falsely low or falsely elevated results. Biotin interference has been shown to occur with various thyroid assays;5-8 parathyroid hormone (PTH) assays;9 fertility hormone assays such as testosterone, progesterone, estradiol, luteinizing hormone (LH), and follicle stimulating hormone (FSH) assays; cancer assays such as PSA; and other assays such as ferritin, folate, cortisol, and vitamin B12.10,11 It was in the context of growing concerns that the FDA issued its November safety communication, which was directed to consumers, healthcare providers, lab personnel and lab test developers. The FDA communication also specified that there has been an increase in the number of reported adverse events, including one death, related to biotin interference with lab tests.
Due to the multiple health benefits that biotin offers, people will continue to take these supplements. Therefore, physicians, lab personnel, and lab test developers need to evaluate practical ways to prevent the interference from biotin. One idea is to make patients wait for hours or days for the biotin to clear from the patient’s body before running lab tests. But the current data suggests that the length of time required to clear biotin from patient samples varies from a few days to a few weeks.10,12 The amount of biotin taken by the patient, how long the patient has been taking biotin (chronicity), and the vulnerability level of each test to biotin interference may add to this challenge. Even on the same diagnostic platform, some tests are more vulnerable to biotin interference than others. As stated in the FDA safety communication, “Currently available data is insufficient to support recommendations for safe testing using affected tests in patients taking high levels of biotin, including about the length of time for biotin clearance from the blood.”13
When waiting-to-clear is clearly contraindicated
This “wait and test” approach certainly will not work in acute care settings, as waiting for biotin to clear from the patient’s system before running critical lab tests is not a feasible option. In the case of patients admitted to the emergency department with chest pain and suspected heart attack, it is important to determine whether they need immediate transfer to the coronary care unit (CCU) or catheterization lab for further care, so it is critical to test troponin levels as soon as possible to help with the diagnosis. While some suspected heart attacks are diagnosed with ECG and clinical findings, in most cases, clinicians rely on a troponin result to help guide patient management. A falsely low troponin result due to biotin interference could lead to grave consequences, including delays in follow-up testing, misdiagnosis, and death.
In cases where patients are suspected of sepsis, measuring procalcitonin (PCT) levels may be extremely useful in patient care and management. But if the PCT test used is vulnerable to biotin interference, it may generate erroneous results that could cause some confusion and possible delays in appropriate treatment initiation. In the case of sepsis patients, every hour of delay in diagnosis increases their mortality by 7.6 percent.14
In pregnant women, tests that are impacted by biotin interference may produce falsely low beta hCG results, which could lead to the erroneous ruling out of early pregnancy, and the pregnant woman could inadvertently be exposed to x-rays and CT scans that may harm the developing fetus. In women who are undergoing in-vitro fertilization (IVF) procedures, determining fertility, timing of ovulation, extraction of eggs, implantation of the embryos, and confirming pregnancy are all precisely scheduled and orchestrated based on the levels of various reproductive hormones such as estradiol, FSH, LH, and beta hCG. If the hormone levels are measured using tests vulnerable to biotin interference, the timing of the various steps in the IVF procedure may not be accurate, which could result in failure of the IVF procedure, and the patient may lose the opportunity to become pregnant.
Education is part of the answer
Will education and awareness efforts about biotin interference solve the problem? Both are critical; however, such efforts cannot provide a complete solution. Patients may not know that the supplements they are taking contains biotin if it is not clearly stated on the label. Since biotin is a supplement, it may not be included in the list of medications taken by the patient. Some patients may know that they are taking biotin, but they may not know that it can interfere with lab tests. As a practical matter, it is not possible for healthcare systems, including hospitals, labs, and doctor’s offices, to know for sure which of their patients are taking biotin and how much.
It is difficult for the lab to identify which patient samples they receive might contain biotin. Patients may take over-the-counter biotin supplements as high as 20 mg per day. Physicians may also prescribe biotin as high as 300 mg per day for conditions such as multiple sclerosis. Therefore, specimens collected from patients taking such high levels of biotin may contain 100 ng/mL to 1,200 ng/mL of biotin, which is why the FDA is recommending that all lab test developers investigate every single assay that uses the biotin-streptavidin technology up to 1,200 ng/mL for biotin interference and communicate the interference they see to the clinical lab community.
Alternative laboratory test options
In this current environment, in which many people are taking biotin and use continues to increase at a steady rate, it is critical for clinical laboratories to have access to alternate testing methods that are not vulnerable to biotin interference. The good news is that not all platforms and not all lab tests are subject to this issue. Only tests that use the biotin-streptavidin “free capture” format are impacted by biotin interference. Assays that use biotin in a “preformed complex” are very unlikely to have biotin interference. There are also alternative methodologies such as magnetic separation technology that are used in some platforms. In this method, magnetic microparticles coated with antigen or antibody are used to capture the targeted analyte, and then they are separated using the magnet. No biotin and streptavidin are used in this method, so biotin in the patient sample does not interfere with the test results.
As noted earlier, the FDA safety communication stated that the data currently available is “insufficient to support recommendations for safe testing using affected tests in patients taking high levels of biotin, including the length of time for biotin clearance from the blood.” Therefore, if a lab uses assays with biotin technology, it is very important to communicate that information to doctors and patients to prevent incorrect test results. The FDA recommends that healthcare providers be aware that many lab tests that use biotin technology are potentially affected, and incorrect test results may be generated if biotin is present in the patient’s specimen. The FDA encourages consumers to educate themselves: to know whether the supplements they take contain biotin. They should let their doctor know that they are taking biotin, and should talk with their doctor about the possibility of biotin interference affecting lab results.
REFERENCES
- Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States: 2003-2006. J Nutr, 2011;141(2):261-266.
- Samarasingh S, Meah F, Singh V, et al. Biotin interference with routine clinical immunoassays: understand the causes and mitigate the risks. Endocrine Practice. 2017;23(8):989-998.
- Nielsen Food Drug Mass (FDM) retail sales data from April 2013-January 2016.
- Peyro Saint Paul L, Debruyne D, Bernard D, Mock DM, Defer GL. Pharmacokinetics and pharmacodynamics of MD1003 (high-dose biotin) in the treatment of progressive multiple sclerosis. Expert Opinion on Drug Metabolism & Toxicology. 2016;12:(3):327-344.
- Barbesino G. Misdiagnosis of Graves’ disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid. 2016;26(6):860-863.
- Brennan JR, Lee SL. High-dose biotin supplement can interfere with common laboratory tests. Endocrine Today. November 2016. https://www.healio.com/endocrinology/thyroid/news/print/endocrine-today/%7B0ff7371d-93a3-4865-b502-60fde9c98122%7D/high-dose-biotin-supplement-can-interfere-with-common-laboratory-tests
- Wijeratne NG, Doery JCG, Lu ZX. Positive and negative interference in immunoassays following biotin ingestion: a pharmacokinetic study. Pathology. 2012;44(7):674-675.
- Palamelai V, Radulescu A, Li D. A case of biotin interference in the Vitros 5600 thyroid-stimulating hormone (TSH) assay. Am J Clin Pathol. 2013;140:427-428.
- Waghray A, Milas M, Nyalakonda K, Siperstein AE. Falsely low parathyroid hormone secondary to biotin interference: A case series. Endocr Pract. 2013;19(3):451-455.
- Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. NEJM. 2016; 375(17):1698.
- Elston MS, Sehgal S, Du Toit S, Yarndley T, Conaglen JV. Factitious Graves’ disease due to biotin immunoassay interference—a case and review of the literature. J Clin Endocrinol Metab. 2016;101(9):3251–3255.
- Mardach R, Zempleni J, Wolf B, et. al. Biotin dependency due to a defect in biotin transport. J Clin Investigat. 2002;109(12):1617-1623.
- U.S. Food and Drug Administration. The FDA warns that biotin may interfere with lab tests: FDA safety communication. November 28, 2017. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm586505.htm
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589-1596.
Ramani Wonderling, PhD, serves as Associate Director of Scientific Relations for Abbott’s Diagnostics Division.