To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
1. List those involved in shared understanding and implementation of caring for those in critical care areas.
2. Discuss the characteristics of the critical care patient and the proper planning of the laboratory environment in nonideal situations.
3. Differentiate critical homeostatic laboratory testing required for the care of critical patients.
4. Discuss back-up laboratory testing for critical care patients in unsuitable environments, such as surge situations or environmental tragedies.
Critical tests versus urgent ones cannot be defined simply by a set of measurands or objective criteria, but on personal understanding of both critical care requirements and laboratory capabilities by laboratorians and caregivers alike. Certain aspects of which can only be found through a working, experiential knowledge. Critical care test portfolios require planning for emergency conditions, since, if critical, certain tests must always be available. Planning for instrument malfunction is basic. Planning for more severe environmental situations that could be possible at your location may be just as critical. Since testing and treatment technology advances are dynamic, continuous re-evaluation and integration with overall goals of an institution and its environment is essential. While core critical and urgent test lists may not change significantly, differences in technology may influence the results leading to the need for more specificity in identifying results on specimens obtained in critical/urgent areas and seamless incorporation into the patient record.
Critical care testing is personal (a few anecdotes)
After a brief stint as a laboratory glassware technician (i.e., dishwasher), the pathologist realized I was a college senior and chemistry major. Shortly thereafter, he decided he needed a new glassware technician, and I would be trained to be a weekend/night technician along with a pre-med senior who had already started. From April through September (full-time), intense training covered more than the college chemistry/biology/physics labs to which we were accustomed. Training included blood, urine, serum, spinal, etc. fluids; chemistries; cell counts/differentials; microscopy; and planting cultures — some automated, but mostly manual methods. No blood gases back then, but we did pH on a Beckman model G, TCO2 on a Natelson, typing and crossmatching, and EKG’s and blood volumes by isotope dilution. It was a hectic summer! After four-and-a-half months of on-the-job training, were the two newbies ready??
Our schedule of alternate weekends and nights in a small hospital gave us a lot of free time to study p-chem or atomic structure — except we mostly spent spare time in the emergency room watching and talking with support and medical staff and helping them out with various tasks, including starting IV’s, restraining patients, etc. There were also times when there was hardly enough time to breathe because something had hit the fan! Incidents such as these:
· An 18-year-old high school track star who was four years younger than me was brought in from the practice field one afternoon, lethargic and near-comatose. I called up to the ER. I had trouble getting blood — it was flowing very slowly into the Vacutainer. I tried a new tube- the same result. Finally, I got enough blood and went back to the lab. I started spinning the hematocrit and began prepping the manual cell count. Looking at the hematocytometer grid – way too many cells! Was the diluent mislabeled? I prepared fresh lysing fluid from scratch. By this time, the hematocrit had finished spinning, and I saw that the buffy coat was huge! Quickly, I looked at the stained differential and immediately called the emergency room and asked the treating doctor to come to the lab. I knew he would not believe me unless he saw it. I still remember the look on Dr B. Shavitz’s face when he looked in the microscope.
· The fourteen eight-year-old kids, walking hand-in-hand to church after school, were mowed down when a driver had an myocardial infarction and died at the wheel. Each child had the same injuries- broken legs and concussions/head-bleeding. All but two were brought into our emergency room on a Friday after the day crew left. Dr. Shavitz and another pediatrician, Dr. R. Abrams were there. A busy night with repeated blood collections, crossmatches, hemaglobin and hematocrit, and area reporters’ and hospital administrator’s questions!!! All the kids survived the night; I went home Saturday at 7:00 AM to hug my kid.
· A 55-year-old pre-surgical man with a leaky (blood) pyloric ulcer. (My father had the same condition and surgeon.) Blood had been crossmatched for the next day’s surgery, and we had 12 units of that type in the bank. But when the patient’s ulcer ‘popped’ at 4:30 that Sunday afternoon, I was alone in the lab. That ulcer did not just bleed fast. The patient nearly exsanguinated, using more than 40 units of blood in the five hours before Dr. Latimer stopped the bleeding.
What critical truly means
These situations all happened in the first months of covering the nights and weekends in the laboratory of a small hospital. The point of these Untold Stories of Critical Care Testing helped in understanding what critical really means and how interrelated testing and direct patient care are in the critical environment. Our experiences — in the lab, at the bedside, and in the field (from urban teaching hospitals to remote settings in Africa and the Amazon) — taught us how crucial it is to break down the silos and stereotypes that too often prevail. (Think 'Abby’ from NCIS or ‘Nurse Ratchet’ from One Flew Over the Cuckoo’s Nest.)
Without a shared understanding between laboratory scientists, clinicians, and caregivers, (and even legislators/regulators), we risk losing what ‘critical’ truly means in patient care. In our view, in-service teaching, training, and continuing education courses must significantly involve the interdependent professions in course design and attendance, including on-the-job training! At a minimum, bench supervisors and those aspiring to those or higher positions must be well grounded in their soft skills. For the characteristics of a critical patient, see Figure 1.
Institutional testing needs: Critical versus urgent
Critical care, for our purposes, relates to the patient as brought into a trauma center in extremis or becomes that way inside the institution for whatever reason. The intervention mantra is Airway, Breathing, and Circulation (ABC). Simultaneously with provision of the initial ‘AB’ by caregivers, laboratory tests for assessing circulation (the ‘C’) and critical underlying pathology are paramount, as is the need to assess/reassess patient status during critical initial care.
Our intent is primarily to point out some considerations that each institution should address when preparing/developing and periodically reassessing critical/urgent test lists and planning for the delivery of needed results. Planning is not ‘one-and-done.’ It must be accomplished in a joint administrative/medical/nursing/laboratory/information technology team, a group with an established and ongoing understanding of the critical and urgent clinical and laboratory environments that can facilitate the evaluation of changing needs.
This team of providers must set the requirements jointly based on established and shared goals for the levels and types of care needed and the technology available. Since critical care is 24/7/365.25, its laboratory support must be as well. Consequently, provision for staffing having broad-based competency must be available or obtainable 24/7/365.25 to support the technology available including routine and back-up technology. The key element is the developed plan and its implementation, not the location for the testing. Then, when a critical/urgent test is required, it is available in a timely fashion even under less-than-optimal conditions.
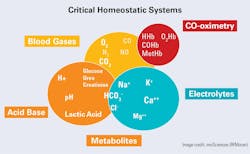
Laboratory oversight: Testing of critical homeostatic systems
Several of this year’s editions of MLO present the tests of the critical homeostatic systems, so they will not be explained here. In summary, these tests consist of measurements reflective of the triad of integrally related metabolic systems that are critical for both the near- and long-term maintenance of life (See Figure 2). While not all tests shown in Figure 2 are always critically needed, it is significant to recognize that CO-oximetry is a requirement in the emergency department and a standout in critical care testing. Even though peripheral or ‘pulse’ oximetry is usually suitable for other critical care areas, it is generally unable to detect dyshemoglobins (e.g., carboxyhemoglobin [COHb] and methemoglobin [MetHb]) necessary for the emergency room. In part due to this common over-reliance on peripheral oximetry, as well as misinterpretation of some of the electrolyte results, we assert that the laboratory must have oversight into all tests performed on body tissues if the results are used for diagnostic or therapeutic intervention.
Blood products and critical/urgent care: Following completion of the laboratory role in the ‘circulation’ part of ABC, type and crossmatch and provision of blood products are obvious items to be included in the critical test environment, as well as the impact of the blood products on the measurands themselves.
Critical and urgent care interface: Immediately following upon and integral with ABC above must be laboratory testing for infectious disease, hematology/immunohematology, coagulation testing, and organ/organ system chemistries. Consider as a criterion that if a physician will make a care decision/action immediately based on the test result and the technology available to perform the test, then the test should be a candidate for your laboratory critical/urgent test list since triaging to the correct care area is an urgent need to ensure the primary areas are ready for the next critical patient.
Infectious disease testing in critical/urgent care: Stat cultures once meant simply planting specimens and reading them later, but innovation—accelerated by COVID-19—has transformed microbial testing. When available, tests including molecular techniques, gas chromatography (GC), mass spectrometry (MS), mathematical transforms, and AI-driven data analysis significantly expedite identification and sensitivity testing. As antimicrobial resistance rises, effective antibiotic stewardship depends on faster, more precise results, which must be in the laboratory’s critical/urgent testing plan. Currently, the tests available rapidly are good but not definitive.
Back-up testing for critical and urgent care
A list suitable for most institutions can be easily prepared to meet testing for critical homeostatic systems. Most important, however, is to consider the need for back up when either specific analytical systems fail, or when other unfavorable conditions arise. If, for example, the blood gas in the emergency room malfunctions can an operational replacement blood gas system elsewhere in the institution be accessed 24/7/365.25, even if it is in another department- make sure that a smooth transition is built into the plan.
Further, plan for both surge situations (COVID) or environmental tragedies, such as those in western North Carolina in 2024 or Kentucky and Tennessee in 2025. Planning for the latter type of disruption can benefit from lessons learned in settings where power outages and lack of clean water are routine challenges. In many parts of Africa, where we have worked, healthcare facilities must adapt and innovate to continue providing essential services despite these constraints. These experiences highlight the importance of resilience, resourcefulness, and contingency planning—principles just as critical in high-resource settings when the unexpected occurs.
The following are some back-up plan suggestions:
General: In disaster situations, several clinical issues are prominent: stress, penetrating trauma, crush injuries, infectious diseases, and ambient toxins such as carbon monoxide or cyanide from fires. What are the critical tests needed to address these clinical situations?
Also, institutional power may be severely limited in quantity and quality. You may need to rely on intermittent generators or battery power or no power. Do you need a separate portable generator for the lab’s basic operational equipment (e.g., blood gases/incubators/refrigeration/microscopy)? What about reagent stability? You may need portable refrigerators and iced coolers.
Chemistry and hematology: Do you have basic colorimeters that can operate reliably on unstable electrical current or battery power? Of the essential tests, are there procedures set up using the reagents at hand to perform the tests under these conditions? Who knows how to make the reagents and do the tests? Are there worries about the FDA and lab-developed test protocols in these conditions? If they are portable, enhanced blood gas systems with electrolytes, urea/creatinine, and glucose may be a viable choice if the system can operate on generator power.
Cell counts (blood, cerebrospinal fluid) using a hematocytometer and white cell differential using Gram stain should be a requirement in back-up planning. Also, your back-up plan should include a spun hematocrit and/or standalone hemoglobin on a simple colorimeter powered by a less-than-electrically-clean generator or a marine battery.
Coagulation: Train emergency staff to use bleeding and clotting times as back up or to validate unexpected results.
Critical organ function tests: While troponin, myoglobin, and creatine kinase are the optimal tests for cardiac assessment, consider testing by less specific methods in emergency situations. For less-than-favorable conditions brought on by environmental disruption, consider the ‘manual’ colorimetric methods that are available and use the colorimeter mentioned above.
Infectious disease tests: If the lab is without electricity, relying on manual techniques, chemical reactions, or visual interpretation become crucial for any back-up plan. Microscopy-based tests (manual staining and examination); rapid diagnostic tests, e.g., lateral flow assays, agglutination, and serological tests (manual visualization/interpretation); and even culture-based tests with minimal electrical requirements can be done (e.g., candle jars or CO2-generating systems).
Conclusions and recommendations
The critical care laboratory in any institution may be uniquely placed in the emergency department, central laboratory, or both. Still, its operation and staffing must be uniquely tuned to what critical means. For critical patients, the testing must take precedence over other laboratory activities and cannot be considered a disruption — it is why we are here. We suggest that the critical care lab be prepared to sustain operations when services are limited. Evaluate what your back-up plan looks like.
Finally, in tune with the latest technology, the results provided by the fully operational critical care lab must be completely comparable to those provided in the central lab, and specimens must be uniquely identified to ensure that there is no ambiguity in the meaning of reported results on the electronic displays throughout the institution and on the electronic health record (EHR) itself.
References
- Moran RF, Grenier RE. The effects of “standard” Blood gas transport and storage conditions on electrolyte results with observations on reported hemoglobin measurement anomalies. In: Methodology and Clinical Applications of Ion Selective Electrodes. Vol 10. ; 1988:57-64.
- Moran RF. Combined Blood Gas, Electrolyte, and total hemoglobin measurements; implication of pre analytical variables on apparent performance of the analyzer. Xes Journees de Biologic Medical. Published online 1989.
- Moran RF. Point-of-care vs central lab "discrepancies": Getting the message across. J Appl Lab Med. 2017;1(5):595-597. doi:10.1373/jalm.2016.021485.
- Moran RF. POC testing and reporting of sodium, and other small molecules need modified IFCC source/type designations to improve operational efficacy and for clinically accurate, unambiguous reporting from LIMS and HIS. EJIFCC. 2023;34(4):271-275.
- Moran RF. Overcoming data management challenges: The biggest challenge of all is….us. Medical Laboratory Observer. January 29, 2024. Accessed March 25, 2025. https://www.mlo-online.com/information-technology/data-management/article/53081209/overcoming-data-management-challenges-the-biggest-challenge-of-all-isus.
- Moran RF. Point-of-care testing — Managing change when you are not in charge. Medical Laboratory Observer. July 22, 2024. Accessed March 25, 2025. https://www.mlo-online.com/continuing-education/article/55094063/point-of-care-testing-managing-change-when-you-are-not-in-charge.
- Moran RF. Use of IFCC/IUPAC format for specimen and test method display in the electronic health record facilitates increased accuracy of information. J Appl Lab Med. 2024:jfae112. doi:10.1093/jalm/jfae112.
- Moran RF. Electrolytes in Blood (and water): Measurement and clinical overview. Medical Laboratory Observer. February 24, 2025. Accessed March 25, 2025. https://www.mlo-online.com/diagnostics/hematology/article/55263357/electrolytes-in-blood-and-water-measurement-and-clinical-overview.
- Point-of-care tests: rapid diagnosis in emergency situations. Medica-tradefair.com. January 6, 2021. Accessed March 25, 2025. https://www.medica-tradefair.com/en/medtech-devices/Point-of-care_tests_rapid_diagnosis_in_emergency_situations.
- Tran NK, Godwin Z, Bockhold J. Point-of-care testing at the disaster-emergency-critical care interface. Point Care. 2012;11(4):180-183. doi:10.1097/POC.0b013e318265f7d9.
- Elrobaa IH, Khan K, Mohamed E. The role of point-of-care testing to improve acute care and health care services. Cureus. 2024;16(3):e55315. doi:10.7759/cureus.55315.
To take the test online go HERE. For more information, visit the Continuing Education tab.