Why your lab should leverage testing with allergen components for sesame allergy analysis
Sesame allergies currently affect roughly one in 200 U.S. citizens and are increasingly becoming a serious public health concern as the number affected continues to rise.1 Given the potential severity and fatality of allergic reactions, and the fact that less than 30% outgrow a sesame allergy, it's a pressing issue that demands attention.1
In response to the rising prevalence of sesame allergies, the Food and Drug Administration (FDA) recently mandated explicit sesame allergen labeling on product packaging.2 The new regulation helps consumers make safer food choices, allowing individuals with sesame allergies to avoid products potentially harmful to them.
While the changes are a big step toward public health safety, it has also introduced challenges for both consumers and diagnostic testing labs. There is a “transition period” as manufacturers adjust to the new labeling requirements, posing a temporary risk for consumers relying on precise allergen information to protect their health. And in the longer term, it puts increasing pressure on labs, as the new rule is likely to trigger an increase in sesame allergen testing as more people seek to understand their potential allergies.
To prepare for the imminent surge in testing requirements, it is vital that labs employ precise and efficient allergy diagnostic tools to streamline their workflows. Additionally, labs must offer the most comprehensive test menu, otherwise clinicians may send their bloodwork elsewhere.
Testing with allergen components — an in vitro blood test that can help determine more precisely which protein a consumer is allergic to — offers deeper insight into a patient’s condition.3 This diagnostic tool is automated and can accurately provide vital details on sesame allergies, enabling labs to meet the demands of an increasingly allergen-aware landscape effectively. This article explores the impact of the new FDA regulations, their potential effects on the lab industry, and how testing with allergen components can help labs rise to these challenges.
Unpacking the FDA’s new act and its effects
Eight major food allergens, including milk, eggs, and peanuts, have historically caused the majority of serious allergic reactions to food in the US.4 In response to the increase in sesame allergies, the government passed the Food Allergy Safety, Treatment, Education and Research (FASTER) Act in 2021, declaring sesame as the ninth major food allergen.5 Effective from January 1, 2023, the FDA requires sesame, alongside the other eight major allergens, to be clearly labelled on packaged foods.
However, food products that were already on shelves, or on their way to shelves prior to 2023, did not need to list sesame on their labels. This causes a precarious transition period, as long-life products on the shelves in 2023 that do not indicate sesame as an allergen might still contain it. Consumers therefore face potential hazards if they rely solely on labelling to make their food choices during this period.
Additionally, as the effects of the new labeling requirements unfold, some manufacturers have begun intentionally adding sesame to their products. By knowingly making sesame-containing products, manufacturers mitigate the requirements and associated costs of proving food products are safe from cross-contamination, which can be expensive and challenging to do. Consequently, certain previously “sesame-safe” restaurants and foods might not be so anymore, and consumers with suspected allergies face unnecessarily restricted diets unless they confirm their sesame allergic status.
Owing to these factors, the labeling shift has escalated the demand for testing with sesame allergen, as increasing numbers of consumers are seeking diagnoses to determine if they can safely eat sesame-containing foods. This surge in testing demand underscores the pivotal role that labs play in providing precise sesame-allergy diagnostic tests.
A closer look at testing with sesame allergen
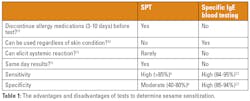
Currently, two main types of tests are employed for measuring sensitization to sesame: skin prick tests (SPT), and blood tests for specific immunoglobulin E (IgE) to whole sesame. SPT measures reactivity of specific IgE bound to mast cells in skin, and blood tests measure specific IgE antibodies in serum.
Each test offers valuable insights, but comes with its respective strengths and limitations, summarized in Table 1.The above tests, while providing useful insight, must be used in conjunction with a thorough clinical history and evaluation of symptoms to make a diagnosis. Furthermore, clinicians should ideally use the tests together to build up a comprehensive picture of an individual's allergy profile.
The most informative way to determine an allergy is through oral food challenges (OFCs). In a sesame OFC, the individual ingests tahini paste in a controlled environment, such as a hospital, and specialists monitor them for any allergic reactions. This is useful to diagnose an adverse reaction to food, but results can be misinterpreted if testing is not done in a masked (or blinded) manner. What’s more, while OFCs are performed in controlled environments to increase safety, there is still a risk of life-threatening anaphylaxis.6 Therefore, OFCs should only be performed when it is acceptable and poses a low risk to the individual.
Clinical history and blood testing with whole allergens are typically used to predict suitability for an OFC, but they are not fully reliable indicators. The need for more precise and comprehensive diagnostic tools, therefore, is critical. Testing with allergen components is one such tool, but what exactly is it?
Testing with allergen components: A breakthrough in sesame allergy detection
The standard allergen blood test is the sIgE test, which considers sesame as a whole. But different proteins present in the allergenic food — allergen components — are associated with the likelihood and potential severity of a clinical allergic reaction, and so it is vital to determine which proteins an individual is sensitized to.7 While blood testing with whole sesame allergen provides useful information, it does not reveal which specific protein a patient is sensitized to, thereby limiting treatment personalization.3
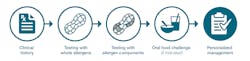
Testing with allergen components determines which specific protein(s) in a food source a patient is sensitized to. As such, it can offer deeper insights into their condition or help determine if there is irrelevant cross-activity. Ultimately, it is a crucial tool to bridge the gap between testing with whole sesame and OFC, helping to predict whether a patient is likely to have a systemic reaction to sesame (see Figure 1). In fact, the FDA recently cleared a sesame allergen component blood test for in vitro diagnostic use, marking a significant advancement in food allergy diagnostics.8The Ses i 1 allergen component test
Ses i 1, a storage protein and major sesame allergen, has emerged as a predictor of sensitization to sesame.9 It is a particularly problematic protein as it is stable to heat and digestion, which significantly elevates the risk of systemic reactions and anaphylaxis.
Patients can now undergo testing for Ses i 1 IgE antibodies, which offers a more complete understanding of their risk for systemic reactions when consuming sesame.7 However, while the test can indicate clinical reactivity, its results should always be interpreted within the context of a patient’s comprehensive clinical history.
A recent study conducted in Japan highlighted the accuracy of Ses i 1 testing, examining 92 sesame-sensitized children divided into symptomatic and asymptomatic groups.7 Upon testing for Ses i 1 sensitization, 92% of the symptomatic group and 32% of the sensitized but asymptomatic group tested positive. The optimal cut-off of IgE level to Ses i 1 was determined to be 3.96 kUA/L, with a remarkable sensitivity of 86.1% and specificity of 85.7%.7
Integrating testing with Ses i 1 allergen component into your profile of tests can provide several benefits:
- Enhance patient care: The approach may help improve the diagnosis of a sesame allergy and optimize patient management.7
- Improved risk stratification: It helps clinicians determine the most suitable candidates for OFC, optimizing resource allocation while ensuring patient safety.3
- More accurate results: Allergen component tests can help distinguish cross-reactivity — for example, between peanuts, tree nuts, and sesame — thereby enhancing the accuracy of diagnoses.3
- Streamline workflows: The incorporation may help to minimize send-out costs and decrease turnaround time for results.
The promising future of testing with allergen components
As FDA regulations shift and the incidence of sesame allergies rise, more and more patients are seeking sesame allergy diagnoses to determine what foods they can safely consume. The importance of providing accurate and reliable allergy testing, therefore, has never been more critical. Testing with Ses i 1 allergen component represents a significant breakthrough in the field, making it easier to accurately determine which patients are suitable for OFC.10
Incorporating such a tool in the laboratory setting not only streamlines the diagnostic process but also substantially improves patient diagnosis and care. By providing more precise results, labs can meet rising testing demands and provide vital support to clinicians and patients alike, ultimately improving healthcare outcomes in the face of increasingly prevalent food allergies.
References
1. Warren CM, Chadha AS, Sicherer SH, Jiang J, Gupta RS. Prevalence and Severity of Sesame Allergy in the United States. JAMA Netw Open. 2019;2;2(8):e199144. doi:10.1001/jamanetworkopen.2019.9144.
2. Allergic to sesame? Food labels now must list sesame as an allergen. U.S. Food and Drug Administration. Published January 26, 2023. Accessed September 27, 2023. https://www.fda.gov/consumers/consumer-updates/allergic-sesame-food-labels-now-must-list-sesame-allergen.
3. Canonica GW, Ansotegui IJ, Pawankar R, et al. A WAO - ARIA - GA²LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013;3;6(1):17. doi:10.1186/1939-4551-6-17.
4. Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA). U.S. Food and Drug Administration. Published November 29, 2022. Accessed September 27, 2023. https://www.fda.gov/food/food-allergensgluten-free-guidance-documents-regulatory-information/food-allergen-labeling-and-consumer-protection-act-2004-falcpa.
5. Congress.gov. Accessed September 27, 2023. https://www.congress.gov/bill/117th-congress/senate-bill/578/all-info.
6. Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3 Suppl 3):S1-148. doi:10.1016/s1081-1206(10)60305-5.
7. Maruyama N, Nakagawa T, Ito K, et al. Measurement of specific IgE antibodies to Ses i 1 improves the diagnosis of sesame allergy. Clin Exp Allergy. 2016;46(1):163-71. doi:10.1111/cea.12626.
8. FDA Clears ImmunoCAPTM blood tests for wheat and sesame allergy. Businesswire.com. Published August 17, 2022. Accessed September 27, 2023. https://www.businesswire.com/news/home/20220817005179/en/FDA-Clears-ImmunoCAP%E2%84%A2-Blood-Tests-for-Wheat-and-Sesame-Allergy.
9. Pastorello EA, Varin E, Farioli L, et al. The major allergen of sesame seeds (Sesamum indicum) is a 2S albumin. J Chromatogr B Biomed Sci Appl. 2001;25;756(1-2):85-93. doi:10.1016/s0378-4347(01)00073-1.
10. Saf S, Sifers TM, Baker MG, et al. Diagnosis of Sesame Allergy: Analysis of Current Practice and Exploration of Sesame Component Ses i 1. J Allergy Clin Immunol Pract. 2020;8(5):1681-1688.e3. doi:10.1016/j.jaip.2019.11.028.
11. Muthupalaniappen L, Jamil A. Prick, patch or blood test? A simple guide to allergy testing. Malays Fam Physician. 2021;31;16(2):19-26. doi:10.51866/rv1141.
12. Direction for Use. ImmunoCAP Specific IgE.
About the Author

Gary Falcetano, PA-C, AE-C
is the U.S. Scientific Affairs Manager for Allergy in ImmunoDiagnostics at Thermo Fisher Scientific. A licensed physician assistant with more than 25 years of diverse experience in emergency and disaster medicine, primary care, and allergy and immunology.