For a printable version of the April CE Story and test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the human leukocyte antigen (HLA) system and how different variants of HLA are associated with specific autoimmune or autoflammatory diseases.
2. Summarize how genetic parameters are now an integral part of routine autoimmune diagnostics using DNA microarrays.
3. Describe the disease characteristics and genomic variants associated with celiac disease, rheumatoid arthritis, ankylosing spondylitis, and psoriasis.
4. Discuss how HLA genotyping plays a role in solid organ transplantation, hematopoietic stem cell transplantation, and transfusion practice for platelet refractoriness patients.15
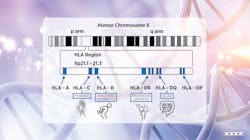
The human leukocyte antigen (HLA) system is a complex of genes responsible for the regulation of the immune system. HLAs feature a high level of genetic polymorphism, which enables the immune system with a selective advantage against the diversity of antigens that a host may be exposed to. Different variants of HLA genes have been associated with specific autoimmune or autoinflammatory diseases such as celiac disease (HLA-DQ2 and DQ8), rheumatoid arthritis (HLA-DRB1), ankylosing spondylitis (HLA-B27), and psoriasis (HLA-CW6). Molecular genetic HLA determination is a tool for the diagnosis and prediction of autoimmune disease risk. The following examples illustrate how genetic parameters are now an integral part of routine autoimmune diagnostics.
HLA-DQ2 and DQ8 in celiac disease
Celiac disease (CD) is an immune-mediated systemic enteropathy triggered by gluten consumption. The genetic risk for CD is related to specific HLA DQ2 and DQ8. HLA-DQ2 and/or -DQ8 markers are present in >98% of CD patients.1 The absence of HLA-DQ2 and -DQ8 markers, due to their high negative predictive value, allows for the exclusion of CD if both markers are not detected. However, the presence of HLA-DQ2/DQ8 alone, is not sufficient to cause celiac disease, as around a third of the healthy population exhibits DQ2/DQ8 alleles.2 Therefore, the value of HLADQ2/DQ8 analysis lies predominantly in exclusion diagnostics.
The role of HLA-DQ2/DQ8 in celiac disease diagnostics has been highlighted in guidelines from the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN).3 Proactive testing for HLA-DQ2 and -DQ8 markers is recommended for screening asymptomatic patients having a family history of CD or other autoimmune diseases. In symptomatic patients, HLA-DQ2 and -DQ8 can be used to confirm CD. Additionally, the detection of these genetic biomarkers can be used to confirm CD in patients with inconclusive biopsy or serology results. For example, in cases where CD patients are on a gluten-free diet, who therefore have very low CD-specific antibody titers, genetic testing is a useful option.3
Using DNA microarray solutions, the identification of the disease-associated alleles HLA-DQA1- and HLA-DQB1, which code for the subunits HLA-DQ2.2, -DQ2.5 and -DQ8, can be done.4 Such microarray testing can differentiate between the homozygous and heterozygous presence of the alleles that code the alpha and beta subunits of HLA-DQ2.2 and -DQ2.5, which enables improved risk assessment in the case of a positive HLA-DQ2 result.4
HLA-DRB1 in rheumatoid arthritis
Rheumatoid Arthritis (RA) is a chronic autoimmune disease that causes joint pain, stiffness, swelling and decreased joint motility and affects an estimated 1.28-1.36 million Americans.5 RA is considered to develop because of interactions between genetic factors and environmental factors. RA is characterized by the production of rheumatoid factors (RFs) and antibodies against citrullinated proteins, or anti-citrullinated protein antibodies (ACPAs). The HLA-DRB1 gene is the strongest known genetic risk factor for RA development and in ACPA-positive RA, the genetic risk is mostly carried by shared epitope (SE)-positive HLA-DR molecules.6 HLA-DRB1 genotypes with HLA-DRB1*04SE (HLA-DRB1*0404, HLA-DRB1*0405, HLA-DRB1*0408), HLA-DRB1*04:01, HLA-DRB1*01 are associated with a high risk for developing ACPA-positive RA.7
Additional antigenic targets have been identified in RA including antibodies against citrullinated -enolase peptide-1 (anti-CEP-1). Anti-CEP-1 antibodies are associated with an erosive disease course, interstitial lung diseases and occur in a subtype of RA associated with smoking and genetic factors.8,9 In a study, antibodies to the immunodominant citrullinated α-enolase CEP-1 epitope were detected in 43–63% of the anti-CCP–positive individuals, and this subset was preferentially linked to HLA-DRB1*04.9
HLA-B27 in ankylosing spondylitis
Ankylosing spondylitis (Bechterew’s disease) is a type of inflammatory autoimmune disease that primarily affects the axial skeleton, tendon insertions, and joints. Ankylosing spondylitis (AS) affects men between the ages of 15 and 30.4,10 Unfortunately, there is no cure for ankylosing spondylitis. Managing pain and symptom alleviation are the only treatments now accessible.10
Tissue antigens, located on the short arm of chromosome 6 of the MHC (Human Major Histocompatibility Complex), are known as HLA. HLA is classified into three classes, one of which is HLA-B, which controls T-cell immunological responses. The class I molecule HLA-B27 is closely associated with ankylosing spondylitis.4,10,11
In 90% of AS patients, HLA-B27 has been identified. However, not all HLA-B27 carriers end up with AS; about 3–6% of HLA-B27 positive carriers develop AS. HLA-B27 can be detected in other conditions such as Reiter’s syndrome, chronic inflammatory bowel disease, inflammatory eye diseases, and various forms of arthritis.10,11
HLA-B27 is currently divided into over 100 subgroups. HLA-B27 detection is crucial in the diagnosis of AS and other rheumatic disorders. It is critical to distinguish between distinct HLA-B27 subtypes because only some of them are linked to AS, while others aren’t.10
The detection of HLA-B27 can be completed using molecular techniques. Particularly for the numerous HLA-B27 subtypes, a PCR approach using allele-specific primers has the potential to deliver trustworthy results. Using the patient’s genomic DNA, molecular detection with DNA microarray technologies can identify HLA-B27 reliably and precisely with just one PCR reaction.4
HLA-Cw6 in psoriasis
Psoriasis is a type of inflammatory autoimmune disease that primarily affects the skin. Other organs, such as the eyes, joints, and vascular system, might also be affected by psoriasis. Unfortunately, people with this ailment are socially isolated and stigmatized, and no cure is currently available.12 Psychological therapy and symptom alleviation are the only treatments now accessible.12,13
The class I molecule HLA-Cw6 is closely associated with psoriasis. HLA-Cw6 has been discovered in 67% of psoriasis sufferers. Psoriasis runs in families in about 40% of instances.4,12 The HLA-Cw6 allele is found in a wide range of people around the world, with White people having a higher frequency than Asians. In Caucasians, the HLA-Cw6 allele raises the likelihood of acquiring psoriasis by a factor of ten. HLA-Cw6 has been linked to psoriasis arthritis. Patients with HLA-Cw6 have a faster onset of psoriatic arthritis, with cutaneous symptoms appearing before musculoskeletal symptoms.13 HLA-Cw6 identification can be distinguished from other types of arthritis, especially in patients who have no family history or unclear skin changes. HLA-Cw6 has been shown to have an impact on illness progression, phenotypic traits, severity, comorbidities, and treatment outcomes on numerous occasions.12,13
DNA microarrays are a proven method for accurately detecting HLA-Cw6.4 Using a blood sample, the HLA-Cw6 gene can be tested. HLA-Cw6 is strongly linked to type I psoriasis vulgaris and psoriasis gutta in 83% of patients and is very weakly linked to type 2 psoriasis vulgaris. HLA-Cw6 positivity is seen in only 44% of patients with type 2 psoriasis vulgaris.12,13
Non- HLA autoimmune diseases The inherited component
Autoimmune diseases are a family of more than 80 chronic illnesses that are often disabling. Autoimmune diseases are characterized by immune system dysfunction leading to the loss of tolerance to self-antigens, presence of increased level of autoantibodies, inflammatory and mediatory cells thereby leading to chronic inflammation.14Multiple studies have been published demonstrating evidence common genetic etiology in ADs. These studies have shown evidence of clustering of multiple autoimmune diseases in families and in individuals, associated with genetic regions predisposing to several autoimmune diseases primarily through GWAS. This genetic overlap is exemplified by the well-known associations of the human leukocyte antigen (HLA) region, and other loci/ SNPs associated with multiple autoimmune disease, such as IL23R, TNFAIP3 and IL2RA. More than 40 regions with evidence for selection and associated with at least one autoimmune disease have been reported. Many of these loci are associated with a single disease, and many are shared between more than one AD such as PTPN22, TNFSF4, ARHGAP31-CD80, TNIP1 and TYK2. Given the complexity of ADs further assessment of variants using functional studies is required to understand the clinical consequences of the association of the genomic variants.
HLA typing in transplantation
The human leukocyte antigen (HLA) system plays an essential role in the regulation of the body’s immune system to counteract pathogens through antigen presentation and the recognition of “self” and “nonself.” HLA matching remains a standard immunologic strategy to determine organ compatibility for recipients. HLA genotyping has a significant role to play in solid organ transplantation (SOT), in hematopoietic stem cell transplantation (HSCT), and in transfusion practice for platelet refractoriness patients.15 The determination of the alloantibodies before transplant is useful for the estimation of risk for antibody-mediated rejection. Crossmatching is used to detect anti-HLA antibodies and allows exclusion of donors with unacceptable HLA antigens. Advancements in tissue typing have introduced HLA matching at the epitope level. HLAMatchmaker (http://www.epitopes.net) is a computer algorithm that determines HLA compatibility between donors and recipients by assessing the 3-dimensional molecular modeling of the epitope-paratope interfaces of antigen-antibody complexes. Even though there are ambiguities with regards to the clinical significance of immunogenic epitopes and identification of immunogenic epitopes remains a work in progress, clinical application of epitope matching in transplantation has been implemented.
HLA and severe cutaneous adverse drug reactions (SCARs)
Severe cutaneous adverse drug reactions (SCARs) are delayed T cell induced hypersensitivity (DTH) including Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), drug reaction with eosinophilic and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP) as described elsewhere.16 These conditions have variation of critical clinical course, skin rashes and severe systemic multi-organ involvement. HLA genotyping for HLA-A*31:01, HLA-A*33:03; HLA-B75 = HLA-B*15:02, HLA-B*15:08, HLA-B*15:11, HLAB*15:21 has been recommended for implementation in routine clinical practice for optimizing safety of medications. The cost-effectiveness of genetic testing, such as screening of HLA, is often an important part of debate especially in the case of reimbursement issues and more studies are being undertaken to demonstrate the benefit.
Conclusion
The identification of specific HLA alleles represents a tool to assess genetic susceptibility to specific autoimmune diseases. Specialized molecular techniques, such as diagnostic microarrays, have been developed for the detection of such disease-associated alleles and have the advantage of high sensitivity and specificity. It is important for the clinician to understand the advantages and disadvantages of different assays such as the most commonly used solid phase assays. As discussed here, molecular techniques offer the identification of HLA alleles related to celiac disease, rheumatoid arthritis, psoriasis, and ankylosing spondylitis.
References
- Cecilio LA, Bonatto MW. The prevalence of HLA DQ2 and DQ8 in patients with celiac disease, in family and in general population. Arq Bras Cir Dig. 2015;28(3):183-185. doi: 10.1590/S0102-67202015000300009.
- Khosravi A, Mansouri M, Rostami-Nejad M, Shahbazkhani B, Ekhlasi G, Kalantari E. The likelihood ratio and frequency of DQ2/DQ8 haplotypes in Iranian patients with celiac disease. Gastroenterol Hepatol Bed Bench. 2016;9(1):18-24.
- Husby S, Koletzko S, Korponay-Szabó I, et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70(1):141-156. doi: 10.1097/MPG.0000000000002497.
- Gosink J. Standardised molecular genetic microarrays for laboratory diagnostics MedLabMagazine. 2012(1). https://www.euroimmun.ch/fileadmin/user_upload/News/Professional-articles/MV_0001_L_UK_A.pdf.
- Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551-1557. doi: 10.1007/s00296-017-3726-1.
- Larid G, Pancarte M, Offer G, et al. In rheumatoid arthritis patients, HLA-DRB1*04:01 and rheumatoid nodules are associated with ACPA to a particular fibrin epitope. Front Immunol. 2021;12. doi: 10.3389/fimmu.2021.692041.
- Balandraud N, Picard C, Reviron D, et al. HLA-DRB1 genotypes and the risk of developing anti citrullinated protein antibody (ACPA) positive rheumatoid arthritis. PloS one. 2013;8(5):e64108-e64108. doi: 10.1371/journal.pone.0064108.
- Liu Y, Liu C, Li L, Zhang F, Li Y, Zhang S. High levels of antibodies to citrullinated ɑ-enolase peptide-1 (CEP-1) identify erosions and interstitial lung disease (ILD) in a Chinese rheumatoid arthritis cohort. Clin Immunol. 2019;200:10-15. doi: 10.1016/j.clim.2019.01.001.
- Mahdi H, Fisher BA, Källberg H, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet. 2009;41(12):1319-1324. doi: 10.1038/ng.480.
- Bowness P. HLA-B27. Annu Rev Immunol. 2015;33:29-48.
- Zeng Y, Hiti A, Moranville S, et al. Human HLA-B27 typing using the BD™ HLA-B27 kit on the BD FACSVia™ system: A multicenter study. Cytometry B Clin Cytom. 2018;94(5):651-657. doi: 10.1002/cyto.b.21630.
- Burlando M, Russo R, Clapasson A, et al. The HLA-Cw6 Dilemma: Is It Really an Outcome Predictor in Psoriasis Patients under Biologic Therapy? A Monocentric Retrospective Analysis. Journal of Clinical Medicine. 2020;9(10):3140. doi: 10.3390/jcm9103140.
- Borroni RG, Costanzo A. HLA-C*06 and psoriasis: susceptibility, phenotype, course and response to treatment. British Journal of Dermatology. 2018;178(4):825-825. doi: 10.1111/bjd.16412.
- RamosP., Shedlock A, Langefeld C. Genetics of autoimmune diseases: insights from population genetics. J Hum Genet. 60, 657–664 (2015). doi: 10.1038/jhg.2015.94.
- Lim WH, Wong G, Heidt S, Claas FHJ. Novel aspects of epitope matching and practical application in kidney transplantation. Kidney Int. 2018 Feb;93(2):314-324. doi: 10.1016/j.kint.2017.08.008. Epub 2017 Oct 20. PMID: 29061333.
- Kloypan C, Koomdee N, Satapornpong P, Tempark T, Biswas M, Sukasem C. A Comprehensive Review of HLA and severe cutaneous adverse drug reactions: Implication for clinical pharmacogenomics and precision medicine. Pharmaceuticals (Basel). 2021;14(11):1077. Published 2021 Oct 25. doi:10.3390/ph14111077.
Ilana Heckler, PhD. As the Scientific Affairs Associate at EUROIMMUN US, Heckler supports scientific collaborations and assists in the validation of diagnostic assays for autoimmunity and infectious diseases.
Azadeh Bojmehrani, PhD is currently the Scientific Affairs Liaison at EUROIMMUN CA. Bojmehrani supports the Canadian team with commercial activities including scientific collaborations and scientific marketing.
Madhuri Hegde, PhD., FACMG is Senior VP and Chief Medical Officer for Global Lab Services at PerkinElmer. Madhuri Hegde is a medical geneticist, a board-certified diplomat in clinical molecular genetics by the American Board of Medical Genetics, and an ACMG Fellow.