Implementation of high-sensitivity cardiac troponin into clinical practice
Earning CEUs
For a printable version of the February CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Recall the history of the troponin assay and the evolution towards a high-sensitive troponin (hs-cTn) assay.
2. Describe the laboratory considerations of the use of the hs-cTn including cutoff concentrations, reference intervals, reporting units and quality control materials..
3. Discuss clinical considerations of hs-cTn testing and the strategies developed for AMI rule out.
4. Describe physician concerns on adopting the use of hs-cTn in the use of diagnosing a cardiac patient correctly.
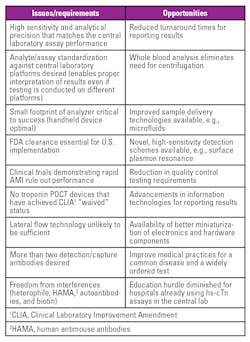
Since 2000, cardiac troponin (cTn) is the preferred biomarker for diagnosis and rule out of acute myocardial infarction (AMI).1 This was reaffirmed with the recent Fourth Redefinition of AMI published in 2019.2 Over the last 25 years, there have been significant improvements in assays for cTn with regards to analytical sensitivity and precision at low concentrations, the area where there is the most clinical value for this test.
High-sensitivity cTnT and cTnI assays have been approved by the Conformité Européene (CE) and commercially available for many years in countries outside the U.S.3 In 2011, the Mitsubishi assay was approved as a near-patient troponin I assay using whole blood.4 In January 2017, the first highly sensitive troponin assay was approved by the US Food and Drug Administration (5th-generation cardiac troponin T, Roche Diagnostics). In the summer of 2018, Beckman and Siemens received FDA approval for the hs-cTnI assay, followed by Abbott in October 2019.
It is appropriate to summarize the steps necessary for a successful launch of these assays with specific reference to clinical laboratories in the U.S. We reviewed the literature that has been generated since the launch of CE-approved assays by current users. Clinical laboratories implementing hs-cTn assays will need to decide on reference ranges, units of measure and assist their emergency department physicians and cardiologists in deciding the optimum frequency of blood collections for patients presenting with chest pain.
Laboratory Considerations
History of cutoff concentrations
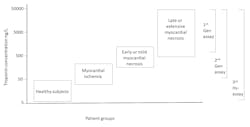
Within a few years, it was recognized that troponin could be used for stratifying the short-term risk of mildly increased troponin. In order to capture these patients, the cutoff concentration was lowered to the value that was associated with an assay imprecision of ≤10 percent. With the release of more sensitive commercial cTn assays, the cutoff was further lowered to the 99th percentile of a healthy population. Thus, troponin evolved from a test to diagnose AMI to one that indicates myocardial injury. As such, the specificity for diagnosis of AMI declined with each generation of assays that demonstrated improved analytical sensitivity.
Today, a troponin assay is defined as “high sensitivity” if it can measure more than 50 percent of healthy patients above the assays limit of detection, i.e., the troponin value that exhibits a 20 percent imprecision.6 In time, hs-cTnT and hs-cTnI will replace all older assays. Will there be a need to develop cardiac troponin assays with even more analytical sensitivity? It is not likely that an assay that can detect troponin below the reference range will produce clinical benefit. There are non-approved research assays that are more sensitive than FDA-approved assays, but the clinical advantages of these assays have not been demonstrated.7
Sex-specific reference intervals
Reference range studies have shown that most hs-cTn assays produce different 99th percentile reference ranges between sexes, with males having higher results than females.8 The FDA requires manufacturers of hs-cTn assays to list separate sex-specific cutoffs in addition to a combined cutoff that encompasses both. Lichtman et al. reported that women suffering AMI were more likely to perceive symptoms as anxiety rather than chest pain.9 Using a lower cutoff value established for women will increase the AMI detection rate among women.
In contrast, experts argue that the treatment of women with mildly increased troponin results may not change, and the use of a combined cutoff at the female limit accomplishes the same goal.10 Until there is international consensus, the clinical laboratory must decide if separate sex-specific reference intervals should be adopted at their institution. These clinical practice guidelines are silent regarding the appropriate cutoff values for transgender patients. If hormonal treatment (estrogens or androgens) does not affect skeletal or cardiac muscle mass, it may be appropriate to use the cutoffs for the individual’s gender at birth.
Reporting units
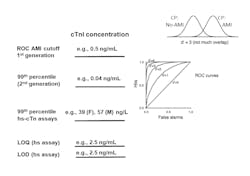
The accepted reporting units for early generations of troponin is ng/mL. A typical cutoff concentration is 0.040 ng/mL. Use of hs-cTnI assays enable detection of values that are ten-fold lower. In order to minimize confusion for interpreting these low values, various international guidelines have recommended converting results to ng/L by multiplying results by 1,000, thereby producing whole numbers.11 For example, a change in serial troponin values from 5 to 10 ng/L is more easily comprehended than reporting a change from 0.005 to 0.010 ng/mL.
Other than conventional troponin assays, there are no clinical laboratory tests where the majority of results are presented as decimals. Laboratories utilizing prior generation assays should retain the older ng/mL units. In this way, values reported in ng/L will necessarily denote the use of a high sensitivity assay. Use of the ng/L value instead of the equivalent pg/mL also conforms with the standards established by International System of Units (SI).
Value assignment for quality control materials
Given that the major advantage of hs-cTn assays is analytical sensitivity, the clinical laboratory must select and validate the appropriate quality control (QC) concentrations. The Academy of the American Association for Clinical Chemistry (AACC) has determined that the first control material should be above the assay’s limit of quantitation and the lowest 99th percentile sex-specific cutoff. The second control material should be close to the highest 99th percentile sex-specific cutoff.12 With this recommendation, the clinical laboratory will need to change the troponin control materials they are currently using, as they are likely to be set at too high of a value. A third control material should be higher to validate the assay’s upper reportable range. This strategy will test medical decision limits for use of hs-cTn assays for rule out and rule in of AMI.
Clinical considerations
Serial troponin testing
The realization of the advantages of using hs-cTn will be to alter the frequency of serial blood collections. Figure 3 illustrates the relationship between troponin assay sensitivity and serial blood collection protocols for AMI diagnosis. A blood collection strategy of zero, six and 12 hours was common with the use of older assays. This was reduced to zero, three and six hours that is currently in place at many medical centers. With the use of hs-cTn assays, emergency departments are reducing the frequency to either zero and one or zero and two hours. It may be more difficult to implement the aggressive one-hour AMI rule out protocol in the U.S. because of the higher numbers of malpractice litigations that occur due to a missed diagnosis of AMI.13
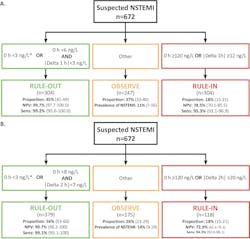
The performance of this accelerated strategy has been demonstrated for a number of hs-cTn assays. Figure 4 shows the specific cutoffs and results using the Siemens hs-cTnI assay (limit of detection 1.6 ng/L, limit of quantitation at 20 percent imprecision 2.5 ng/L). The APACE study was conducted in 12 centers across five European countries with enrollments of over 1,000 patients and had an adjudicated AMI rate of 18 percent.14 Using an algorithm that collects blood at zero and one hour, and zero and two hours, the negative predictive value (NPV) was 99.7 percent with 50 percent of subjects that can be safely discharged. There are other studies demonstrating similar performance using other commercial assays for hs-cTnI and hs-cTnT.15,16
Single troponin testing for AMI rule out
The data on Figure 4 shows that AMI rule out can be achieved with either serial testing using absolute (i.e., <8 ng/L) and delta change (i.e., <7 ng/L) cutoffs at two hours, or the results of a single low troponin value using a lower cutoff concentration (<3 ng/L).14 The performance of using the admission sample troponin result alone has also been examined. In order to achieve comparable performance to the dual cutoff strategy, Body et al.17 and Sandoval et al.18 lowered the hs-cTnT and hs-cTnI cutoff concentrations at the assay’s limit of detection (LoD) and reported high NPV for AMI rule out (>99th). The number of patients that could be ruled out was also comparable to the dual cutoff strategy. Figure 4 shows the cutoff of <3 ng/L for discharge using the admission sample alone.
AMI rule out at the time of presentation is a very aggressive strategy, and few hospitals have adopted it worldwide. The risks of a missed AMI must be weighed against the improvement in emergency department patient workflow. The success can be improved if other important factors are considered when making early discharge decisions, e.g., the presence of low clinical risk scores such as thrombolysis in myocardial infarction (TIMI) or APACE,19 the absence of any myocardial ischemia on the electrocardiogram or if history of chest pain onset from symptom onset is greater than three hours. In the U.S, the risk of a missed AMI is lower than in European countries, as the AMI rule in rate at 5-8 percent is lower than what is observed in other parts of the world. Unfortunately, the FDA does not permit clinical laboratories to report hs-cTn values down to the limit of detection. Therefore, this admission sample rule out strategy where the cutoff is at the LoD cannot be enacted today.
Issues for cardiologists
While there are major advantages for ruling out AMI earlier from the ED, there are real concerns by the cardiologists that adopting a lower cutoff concentration with cardiac troponin will lead to an increased admission of patients who have an increased hs-cTn result but are not suffering from an AMI. It is essential that ED physicians and cardiologists understand how to interpret increased hs-cTn values within the context of the history and presentation. The use of the hs-cTn assay will result in an increased number of patients having an abnormal level.
There are a number of etiologies for increased troponin, including heart failure, renal insufficiency, sepsis, cardiac and non-cardiac surgery and venous thrombosis. Each of these are serious medical conditions that warrant careful examination with possible hospitalization. In addition, risk stratification studies have consistently shown that patients with an increased cardiac troponin, outside of the context of acute coronary syndromes, are at higher risk for adverse cardiac events than patients with the same disease but without an abnormal troponin.20
Therefore, medical attention for these patients is warranted. However, it is not appropriate to send these individuals for acute revascularization (e.g., angioplasty). Some physicians may argue that some of these diseases do not fall under the jurisdiction of cardiology. The result of serial measurements is an essential criteria to determine if a patient with a positive troponin has active or chronic cardiac injury, with unchanging values favoring the latter. If a rising and falling pattern of troponin is observed, acute cardiac injury is suspected, but a diagnosis of AMI is not absolute. For example, acute exacerbation of heart failure can produce a transient troponin increase that is not caused by acute coronary syndromes.
Conclusions
In order to reap the benefits of hs-cTn assays, a medical practice must first, adopt the 99th percentile cutoff strategy and second, understand that diseases besides AMI will produce abnormal results. Prior to implementation, it is essential that members of the emergency department, cardiologists and clinical laboratory meet to discuss the ramifications of hs-cTn assays. There may be confusion with the change of units and use of sex-specific cutoff limits.
Past experience has shown that there will be an increase in unnecessary admissions of patients as the direct result of reporting low-level positive results. The objective of hs-cTn assays is to reduce the time for AMI rule out and improve the accuracy of AMI diagnosis and risk stratification for future adverse cardiac events in the short term. Therefore, it will be important to conduct audits of patient admission and discharge to determine appropriateness and to educate the staff making these decisions.
For a printable version of the February CE test go HERE or to take test online go HERE. For more information, visit the Continuing Education tab.
References
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969.
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infraction (2018). Eur Heart J 2019;40(3):237-69.
- Christenson RH, Jacobs E, Uettwiller-Geiger D, et al. Comparison of 13 commercially available cardiac troponin assays in a multicenter North American Study. J Appl Lab Med. 2017;1(5):544-561.
- Christenson RH, Mullins K, Duh SH. Validation of high-sensitivity performance for a United States Food and Drug Administration cleared cardiac troponin I assay. Clin Biochem. 2018;56(1):4-10.
- Hochhlozer W, Morrow DA, Giugliano RP. Novel biomarkers in cardiovascular disease: update 2010. Am Heart J 2010;160(4):583-94.
- Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem. 2010;57:1303–1306.
- Wu AHB, van Wijk XM. A novel ultra-high sensitivity tropoinin I assay for chest pain patients with no evidence of troponin I using a conventional assay. Clin Biochem. 2014(4-5);41:358-359.
- Gimenez MR, Reiter M, Twerenbold R, et al. Sex-specific chest pain characteristics in the early diagnosis of acute myocardial infarction. JAMA Intern Med. 2014;174(2)241-249.
- Lichtman JH, Leifheit EC, Safdar B, et al. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction. Circulation 2018;137(2):781-790.
- Giannitsis E. Potential concerns regarding the use of sex-specific cutpoints for high-sensitivity troponin assays. Clin Chem. 2018;63(1):264-266.
- Apple FS, Jaffe AS, Collinson P, et al. International Federation of Clinical Chemistry (IFCC) Task Force on Clinical Applications of Cardiac Biomarkers. IFCC educational materials on selected analytical and clinical applications of high-sensitivity cardiac troponin assays. Clin Biochem. 2015;48(4-5):201-203.
- Wu AHB, Christenson RH, Greene DN, et al. Clinical laboratory practice recommendations for use of cardiac troponin in acute coronary syndrome: expert opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardio Biomarkers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. 2018;64(4):645-655.
- Moy E, Barrett M, Coffey R, et al. Missed diagnoses of acute myocardial infarction in the emergency department: variation by patient and facility characteristics. Diagnoses 2015;2(1):29-40.
- Boeddinghaus J, Twerenbold R, Nestelberger T, et al. Clinical validation of a novel high-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin Chem. 2018;64(9):1347-1360.
- Reichlin T, Cullen L, Parsonage WA, et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Am J Med. 2015;128(4):369-375.
- Boeddinghaus J, Reichlin T, Cullen L, et al. Two-hour algorithm for triage toward rule-out and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin I. Clin Chem. 2016;62(3):494-504.
- Body R, Twerenbold R, Austin C, et al. Diagnostic accuracy of a high-sensitivity cardiac troponin assay with a single serum test in the emergency department. Clin Chem. 2019;65(8):1006-1014.
- Sandoval Y, Smith W, Shah ASV, et al. Rapid rule-out of acute myocardial injury using a single high-sensitivity cardiac troponin I measurement. Clin Chem. 2017;61(1):369-376.
- Hollander JE. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation 2016;134(7):547-564.
- Eggers KM, Jernbert T, Lindahl B. Cardiac troponin elevation in patients without a specific diagnosis. J Am Coll Cardiol. 2019;73(1):1-9.