Preanalytical errors and critical variables in POCT
Today’s “smart” technology enables us to have important information at our fingertips. Point-of-care testing (POCT)—also referred to as “near patient, bedside, and extra-laboratory testing”1—offers the rapid delivery of healthcare information as well.
Centralized laboratory testing was the standard until the mid-1980s. Since that time, many laboratory tests (e.g., glucose and blood gas testing) have transitioned to patient care settings, including physicians’ offices, ambulances, and hospital units (e.g., the intensive care unit, emergency department, surgical suites), as well as clinics, dialysis centers, and nursing homes.2 Devices for POCT range in size from small handheld meters for glucose monitoring to larger benchtop analyzers for hematology.
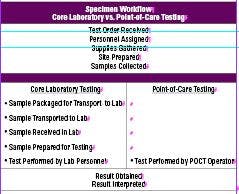
The surge in POCT may be attributed to distinct advantages over core laboratory testing, fewer sample workflow steps (Table 1), faster test turnaround time, more timely triage or treatment, potential decrease in hospital length of stay, and lower sample volume requirements.
A quick result does not always mean an accurate result, however. As in the core laboratory, the preanalytical phase of the POCT process may introduce errors that could impact test results. Errors in POCT can be particularly problematic if immediate action is taken based on incorrect results obtained. Consider the consequences for a patient who receives a higher dose of warfarin based on an inaccurate test result. Receiving an excess amount of warfarin can lead to cerebral hemorrhage or gastrointestinal bleeding. Additionally, if repeat testing is necessary as a result of the incorrect result, the time for obtaining diagnosis and initiating treatment may be prolonged, negating the benefits of POCT testing. This is detrimental for critical care patients who often undergo frequent testing to monitor their condition (e.g., blood gases, glucose, hemoglobin), reaffirming the importance of quality specimens. Collecting quality specimens may be a challenge in POCT, since testing is performed outside the control of the laboratory, often by less-skilled, non-laboratory personnel, and in different clinical settings. Understanding some of the preanalytical factors that can impact POCT results goes a long way in ensuring that the results obtained are valid ones.
Patient preparation
Proper identification is of crucial importance. As in the core laboratory, accurate test results begin with correct patient identification. Not all POCT devices have built-in barcode scanners to facilitate bedside patient identification, which requires manual entry of the patient’s information and increases the potential for transcription error.3 Operators must also be cognizant of similar patient surnames (Johnson vs. Johnston) and dates of birth (1/1/55 vs. 1/11/55). Verification of patient identification should occur at the bedside prior to testing. Additionally, at least two patient identifiers should be used as recommended by the Joint Commission and Clinical Laboratory and Standards Institute (CLSI).4-6
Sample preparation
Use the appropriate anticoagulant. Heparin is the primary anticoagulant for testing blood gases, ionized calcium, and electrolytes. However, the amount of heparin may influence test results. Higher concentrations of liquid heparin may dilute the sample and alter the values of pCO2, pO2, and Ca++. If liquid heparin is used, the ideal volume should be less than 10 percent, preferably five percent or less of the total sample volume.7,8 Alternatively, a balanced formulation of dry lithium heparin could be utilized to measure blood gases and electrolytes.9
In addition, tubes should be filled to the specified level by the manufacturer to prevent excess anticoagulant-to-blood ratio. Tubes that are overfilled will not have sufficient anticoagulant to prevent clotting.
Eliminate air bubbles. Air bubbles in arterial samples may cause a bias in blood gas measurements, particularly for pCO2 and pO2. Any air bubbles should be expelled by gently tapping the sides of the syringe.
Mix well. Inadequate mixing of the specimen may cause incomplete dissolution of the anticoagulant in the syringe and the formation of clots, which may negatively impact hematocrit and obstruct the analyzer probe. Mixing should be accomplished immediately after collection by inverting the syringe a minimum of five times and then rolling the syringe between the palms for five seconds.10 Vigorous mixing or agitation may cause the specimen to hemolyze.
Prevent hemolysis. Squeezing or milking the puncture site for capillary collection may contaminate the specimen with tissue fluid and cause hemolysis, which could interfere with test results (e.g., elevations in potassium, troponin, pCO2). Since visible hemolysis is not apparent in whole blood specimens, any inconsistent results should be repeated to verify accuracy.11
Store properly. Operators should consult the recommendations provided by the manufacturer for proper storage conditions. Storing cartridges beyond these recommendations may affect test results. For instance, exposure of glucose test strips to air, light, and humidity may alter their performance.
It is important to note that whole blood specimens continue to metabolize after collection, warranting prompt analysis to avoid changes in blood gases and related parameters.11,12
Training all users
To minimize the risk of errors, standard operating procedures should be established which detail best practices for each POC test on each instrument. These procedures should be coupled with continuous training for all users to ensure comprehension and competence.
Point-of-care testing is expected to increase exponentially, with the addition of more assays and instruments. As in the core laboratory, a quality preanalytical phase in POCT is key to a quality specimen. Understanding the preanalytical factors that may contribute to errors can help healthcare professionals maximize the potential of POCT for their patients.
References
1. Price C. Point of care testing. BMJ. 2001;322:1285-1288.
2. Dubois J. The role of POCT and rapid testing. MLO.2013;45(9):18-22.
3. Shaw J. Practical challenges related to point of care testing. Pract Lab Med. 2016;4:22-29.
4. National Patient Safety Standards from the Joint Commission
5. POCT-07-A. Quality management: Approaches to reducing errors at the point of care; approved guideline. Clinical Laboratory and Standards
Institute, 2010.
6. Alreja G, Setia N, Nichols M, Pantanowitz L. Reducing patient identification errors related to glucose point of care testing. J Pathol Inform. 2011:2:22.
7. Higgins C. The use of heparin in preparing samples for blood gas
analysis. MLO. 2007;39(10):16-20.
8. Chhapola V, Kumar S, Goyal P, Sharma R. Use of liquid heparin for blood gas sampling in pediatric intensive care unit: a comparative study of effects of varying volumes of heparin on blood gas parameters. Ind J Crit Care Med. 2013;17(6):350-354.
9. Ancy JJ. Blood gases and preanalytic error prevention. RT Magazine. February 7, 2012.
10. Narayanan S. Preanalytical issues related to blood sample mixing
11. Nichols J. Risk management for point of care testing. J Inter Fed Clin Chem Lab Med. 2014;25(2):154-161.
12. Dukic L, Kopcinovic L, Dorotic A, Barsic I. Blood gas testing and
related measurements: National recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem Med. 2016;26(3):318-336.