CONTINUING EDUCATION
To earn CEUs, see current test at
www.mlo-online.comunder the CE Tests tab.
LEARNING OBJECTIVES
Upon completion of this article, the
reader will be able to:
- recognize personnel requirements for ZMC’s Biosafety Level 3+ lab;
- recognize the biological agents that ZMC handles, and the tests and
processes performed on these agents to make them available for outside
use; - discuss the next probable pandemic predicted by ZMC and the
rationale for the choice; and - recognize structures and genetics of influenza viruses,
incubation period for SARS, and disease and death rates for
influenza and HIV.
Systemic lupus erythematosus (SLE) is the
most common of the autoimmune disorders and can involve virtually any organ
in the body. SLE is associated with pleuropulmonary manifestations in well
over 50% of cases.1 The clinical spectrum ranges from mild,
self-limited, pleuritic chest pain to fulminant and rapidly fatal pulmonary
hemorrhage. Pleuritis, with or without pleural effusion, is the most common
manifestation and can be particularly troublesome to detect and manage.
Pleuropulmonary problems may contribute significantly to overall mortality
in SLE.2
Clinical history
In our clinical setting, we encountered a 42-year-old African-American
male who presented with acute shortness of breath and new facial rash, which
was associated with substernal, dull, intermittent, and non-radiating chest
pain. Patient had experienced fever and 10-pound weight loss during this
time with a past history of congestive heart failure, hypertension, chronic
renal insufficiency, hypothyroidism, and anemia of chronic disease. He
related a history of untreated SLE, and was believed to be in remission for
many years due to lack of joint involvement. Recurrent pneumonia with
pleuritis requiring previous hospital admissions had occurred in the past
year.
Posteroanterior chest radiograph shows bilateral pleural effusions with
bilateral atelectasis.
On examination, the patient was in mild distress with diffuse rhonchi in
bilateral lung fields. A chest radiograph demonstrated bilateral alveolar
and interstitial opacities, blunting of the costophrenic angles bilaterally,
and bilateral pleural effusions. Impression was bilateral pneumonia with
stable pulmonary edema and bilateral pleural effusion (see Image 1).
Worsening dyspnea necessitated surgical drainage of the pleural effusion.
Approximately 500 mL of serosanguinous fluid was removed and sent for
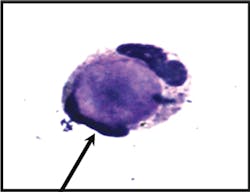
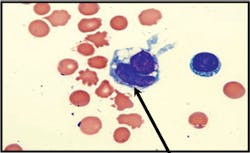
laboratory evaluation. On examination of a Wright-Giemsa stained cytospin
preparation, a single lupus erythematosus (LE) cell was identified,
characterized by homogenous nuclear material engulfed by a neutrophil (see
Image 2a). The cell was present in a background of numerous segmented
neutrophils, lymphocytes, plasma cells, and macrophages.
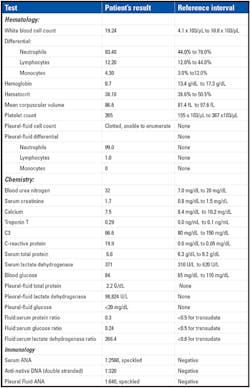
A summary of laboratory values is given in Table 1.
Discussion
Image 2aLE cell: Wright-stained smear of the pleural fluid shows a typical single LE
cell consisting of a neutrophil with flattened nucleus engulfing a central
paler homogenous mass.
Image 2b
Tart cell: A typical tart cell, which is smaller and has a non-homogenous
(clumped) appearance in contrast to the smooth homogenous character of the
hematoxylin bodies in true LE cells.
This patient presented with substernal chest pain and shortness of
breath, accompanied by productive cough and fever, typical of lupus
pleuritis. Several diagnostic possibilities exist when a patient presents
with acute pleuritis manifesting as severe chest pain, fever, dyspnea, and
pleural effusions. The clinician must consider pulmonary embolus, viral
infection, parapneumonic effusion, tuberculosis, congestive heart failure,
and collagen vascular disorders — such as SLE or rheumatoid arthritis — in
the differential diagnosis. Chemistry analysis revealed the fluid to be an
exudate by Light criteria, with very high lactate dehydrogenase (LDH) and
elevated-fluid-to-serum-LDH ratio, rather than a simple transudate due to
congestive heart failure.3 Pleural effusion due to lupus
pleuritis is typically an exudate and may be unilateral or bilateral. In
most cases the glucose is >60 mg/dL and the complement levels are frequently
low. When an LE cell is seen in pleural fluid, and this finding correlated
with immunologic studies for SLE, the observation can be helpful in
establishing lupus pleuritis.
Results of the pleural-fluid cell count included the finding of the LE
cell. Subsequent pleural-fluid and serum antinuclear-antibody (ANA) tests
were performed, along with other immunologic tests that confirmed the
diagnosis of SLE. Intravenous steroid therapy was initiated, after which the
bilateral pleural effusions dramatically improved. Serous effusions as a
result of SLE tend to be more common in the chronic stage of the disease,
and the presence of LE cells in an effusion is associated with the presence
of active disease.
LE cells are neutrophilic phagocytes that contain intracytoplasmic
hematoxylin bodies. The hematoxylin bodies are thought to be formed by the
opsonization of cells by ANA typically found in SLE patients. These
antibodies lead to the denaturation of dead or injured cells, forming
homogenous oval-shaped bodies that are referred to as “hematoxylin bodies”
because they stain blue with common cytologic stains such as Wright-Giemsa,
Papanicolaou, and hematoxylin and eosin stains. The hematoxylin bodies are
engulfed by neutrophils, creating LE cells. In cytologic preparations, LE
cells must be distinguished from “tart cells” or “pseudo-LE cells,” which
result from the phagocytosis of nuclear debris by macrophages, rather than
neutrophils, and are generally seen in effusion fluid — independent of the
cause of the effusion. The phagocytosed debris within the tart cell is
smaller, and has a non-homogenous (clumped) appearance in contrast to the
smooth homogenous character of the hematoxylin bodies in true LE cells (see
Image 2b). LE cells are also seen in bone-marrow aspiration material,
synovial fluid, cerebrospinal fluid, and pericardial fluid of SLE patients.
The presence of LE cells in any of these specimens, in conjunction with the
appropriate clinical picture and laboratory values, would contribute to the
diagnosis of SLE.
The features that, in the past, were most helpful to distinguish lupus
pleuritis from other causes of effusions were immunologic, such as reduced
levels of complement, presence of ANA, and LE cells.4
Various studies, however, have shown that neither reduced levels of
complement nor the presence of ANA in pleural fluid is a sensitive or
specific predictor of lupus pleuritis.
First,
complement level may be decreased in the fluid and is helpful in
differentiating effusions caused by connective-tissue disease from those
resulting from other causes of pleural effusions but is not specific to
lupus pleuritis — although reduced complement values have been reported in
SLE. Hunder, et al, suggested that pleural-fluid complement depletion may be
secondary to immunologic activation and that immune complexes probably
contribute to the development of pleuritis in lupus.5
Second, presence of ANA in pleural effusion has been suggested to be a
sensitive and specific marker for active pleurisy due to SLE.6 In
a series of 100 patients with pleural effusions of unknown etiology, the ANA
was positive in seven patients with SLE and in one patient with drug-induced
LE but not in patients with other diagnoses. In a study of 18 patients with
SLE and pleural effusions, however, two of the patients with pleural
effusions from causes other than lupus had ANA in the pleural fluid,
although in lower titer compared to patients with lupus pleuritis. Khare, et
al, in a study of 82 patients with pleural effusions, found a higher
false-positive rate (10.8%) when using pleural-fluid ANA to diagnose lupus
pleuritis.7 A helpful feature was that a lower pleural-fluid ANA
titer was seen in the majority of the patients without lupus pleuritis,
making higher titers of pleural-fluid ANA (>1:160) more suggestive of lupus
pleuritis. A pleural-fluid-to-serum-NA ratio of 1.0 or more is reported to
be strongly suggestive of lupus pleuritis. The finding is not a constant in
lupus pleuritis, however, as Khare, et al, reported, only three of eight
patients with lupus pleuritis had a pleural-fluid-to-serum-ANA ratio of 1.0
or more.7
The features that, in the past, were most helpful to distinguish
lupus pleuritis from other causes of effusions were immunologic, such as
reduced levels of complement, presence of ANA, and LE cells.
Similarly, Wang, et al, showed only five of 10 patients with lupus
serositis had a pleural-fluid-to-serum-ANA ratio of 1.0 or more.8
Although the ratio was less than 1.0 in patients with SLE who had effusions
from other causes, Wang, et al, found that 10 of 13 non-SLE patients with
high ANA titers also had ratios of 1.0 or higher. These findings suggest
that pleural-fluid-to-serum-ANA ratio has no additional value over the ANA
to identify lupus pleuritis in patients with high-effusion ANA titers.
Different patterns of ANA immunofluorescence were first noted by Beck, et
al, in 1961.9 Khare, et al, suggested that the homogenous
staining pattern in pleural fluid was predominantly found in patients with
lupus pleuritis, and a speckled staining pattern in pleural fluid suggested
an alternative diagnosis.7 Wang, et al, however, found that only
five of 10 patients with lupus serositis had a homogenous staining pattern,
and the other five had a speckled pattern. Furthermore, seven of 35 non-SLE
patients had a homogenous pattern. These observations suggest that a reduced
level of pleural-fluid complement, pleural-fluid-to-serum- ANA ratio, and
homogenous ANA staining pattern are neither sensitive nor specific
predictors of lupus pleuritis, and results of these laboratory parameters
require correlation with patient presentation and other laboratory
immunologic findings.
Conclusion
Image 3Papanicolaou-stained preparation showing numerous LE cells with
multilobed nuclei and a hematoxylin body. The LE cells on Papanicolaou-stained
preparations are smaller and show less nuclear compression of the
neutrophilic phagocyte by the hematoxylin body in comparison to the
appearance on Wright-Giemsa.
This case demonstrates that the cytologic examination of pleural fluid is
important as a diagnostic study. The finding of the single LE cell was
helpful in establishing the previously unsuspected diagnosis of lupus
pleuritis, so that beneficial treatment could be initiated. In the past, a
test was performed by the laboratory for the identification of LE cells and
was known as the LE preparation. This test was considered specific for SLE,
simple to perform, and recommended when the cause of pleural effusion was
unknown. Advanced immunologic assays for SLE have replaced the labor
intensive and insensitive LE preparation, but LE cells may still be
encountered in preparations for fluidcell counts or cytopathology
examination (see Image 3). Nevertheless, recognition and reporting of these
cells, when found incidentally, can still be valuable as illustrated in this
case of laboratory microscopic lights and radiographic imaging shadows.
Purva Gopal, MD, MS;
Santosh K. S. Math, MD, MS; and
Sandra C. Hollensead, MD, are all associated with the Department of
Pathology and Laboratory Medicine at University of Louisville School of
Medicine in Louisville, KY.
References
1. Mulherin D, Bresnihan B. Systemic lupus erythematosus.
Baillieres Clin Rheumatol. 1993;7:31-57.
2. Brasington RD, Furst DE. Pulmonary disease in systemic lupus
erythematosus. Clin Exp Rheumatol. 1985;3:269-276.
3. Light RW. Pleural Effusions: The Diagnositc Separation of Transudates and
Exudates. Ann Intern Med. 1972;77::507-513.
4. Good JT Jr, King TE, Antony VB, Sahn SA. Lupus pleuritis. Clinical
features and pleural fluid characteristics with special reference to pleural
fluid antinuclear antibodies. Chest. 1983;84:714-718.
5. Hunder GG, McDuffie FC, Huston KA, Elveback LR, Hepper NG. Pleural fluid
complement, complement conversion, and immune complexes in immunologic and
nonimmunologic diseases. J Lab Clin Med. 1977;90:971-980.
6. Leechawengwong M, Berger HW, Sukumaran M. Diagnostic significance of
antinuclear antibodies in pleural effusion. Mt Sinai J Med.
1979;46:137-139.
7. Khare V, Baethge B, Lang S, Wolf RE, Campbell GD Jr. Antinuclear
antibodies in pleural fluid. Chest. 1994;106:866-871.
8. Wang DY, Yang PC, Yu WL, Shiah DC, Kuo HW, Hsu NY. Comparison of
different diagnostic methods for lupus pleuritis and pericarditis: a
prospective three-year study. J Formos Med Assoc. 2000;99:375-380
9. Beck JS. Variations in the morphological patterns of “autoimmune”
nuclear fluorescence. Lancet. 1961;1:1203-1205.
MLO’s
Continuing Education Test is available online only.
Print out and mail a copy with your check, or use the new online CE test
and convenient online payment feature available through the auspices of
Northern Illinois University.
Go to www.mlo-online.com and
look under CE Tests.